Prostate cancer is the most prevalent type of cancer in men. In 2005, 3 662 Norwegian men were diagnosed with this cancer; median age at the time of diagnosis was 71 years and 12.2 % were younger than 60 years (1). Mortality has been unchanged the last 10 years in Norway - and is the highest in Europe.
The prognosis for men with prostate cancer is related to several clinical factors - findings in rectal exploration (TNM classification: T: extent of primary tumour, N: occurrence of lymph metastases, M: occurrence of distant metastases), prostate-specific antigen (PSA) in blood and Gleason score; i.e. the growth pattern of tumour tissue found in biopsies. Based on these factors patients with prostate cancer are divided into prognostic risk groups (Box 1) (2). The choice of treatment currently depends on assessments done by the urologist and oncologist, age, comorbidity, hospital routines and patient wishes.
Box 1
Biochemical control after curative surgery or radiation therapy by risk group
-
Low risk: All prognostic criteria present
T1C-T2B, PSA-level ≤ 10 µg/L, Gleason-score ≤ 6
5 years of biochemical control: 90 %
-
Intermediate risk: One criterion present
T2C-T3B, PSA-level > 10 µg/L or Gleason-score >= 7
5 years of biochemical control: ca. 70 %
-
High risk: Two or more criteria present
T2C-T3B, PSA-level > 10 µg/L or Gleason-score >= 7
5 years of biochemical control: ca. 50 %
Surgery and radiation therapy are the two treatment modalities considered to have a curative potential for patients with local or locally advanced prostate cancer. In the low risk group, prostatectomy and radiation therapy are considered to be equally efficient. For patients with more aggressive disease it is important to use treatment with a better prognosis and fewer side effects. Radiation therapy is frequently offered to patients in the high-risk group or to those with serious comorbidity irrespective of prognostic group, often in combination with androgen deprivation therapy.
Several studies have shown an association between radiation dose to the prostate and tumour control (3 - 5). For patients with PSA levels > 10 µg/L, an increase of external radiation dose to the prostate is shown to give both longer recurrence-free time-periods and an increased cure rate; shown in preliminary results from the first prospective, randomised dose escalation study (Pollack and collaborators 2000) (6). However, higher radiation doses can give long-term side effects that may impair quality of life. The most important limiting factor for giving a full dose of external radiation is normal tissue toxicity in surrounding organs, such as the urinary bladder and rectum. With brachytherapy (brachy - greek for short), radioactive sources with relatively short irridation distance (irridation [192Ir]) are brought directly into the organ to be radiated. High-dose-rate brachytherapy combined with external radiation is a method which increases the radiation dose to the prostate without increasing the dose to the surrounding organs. This has not been an established treatment method in Norway up to now, but the method has been used for 10 - 15 years (7 - 9) in other countries.
The aim of this article was to describe the treatment method (high-dose-rate brachytherapy in combination with external beam radiation) and our experience with the first 100 patients with prostate cancer treated in Norway.
Material and method
High-dose-rate (high radiation dose per time unit) brachytherapy is a hi-tech radiation method in which thread-formed radiation sources are temporarily inserted into the prostate. The source is introduced through hollow steel needles that are inserted through the perineum and into the gland.
On the day of treatment the patient has a catheter in the urethra and has started antibiotic prophylaxis. The patient is given a bulk laxative, placed in a gynecology position and is sedated. An ultrasound probe in rectum enables visualisation of the prostate gland. An air-gel mixture is injected into the catheter for clear delineation of the urethra. The prostate is lifted up by a water-filled condom around the ultrasound probe. Depending on the size of the prostate, 12 - 18 hollow steel needles are inserted through the perineum and into the prostate under ultrasound guidance.
The ultrasound images with the needles in place are transferred electronically to the treatment planning system. A treatment plan is developed based on the needles’ real position (fig 1). The completed plan, with pre-calculated times for radiation exposure in each needle, is transferred to the treatment apparatus (MicroSelection). Before insertion of the radiation sources, the water is taken out of the condom so the rectal mucous membrane falls down dorsally and away from the prostate. An iridium source (192Ir) is thereafter inserted into every needle (fig 2). A high radiation dose (at least 10 Gy) is emitted quickly into the core of the gland (gray, unit for absorbed radiation dose). The radiation does not reach far, so a dose reduction is quickly obtained outside of the prostate capsule. This is best for the surrounding organs, especially rectum. Urethra is an organ at high risk of toxicity and the maximum dose against urethra should be < 12 Gy. The entire procedure takes 2 - 3 hours.
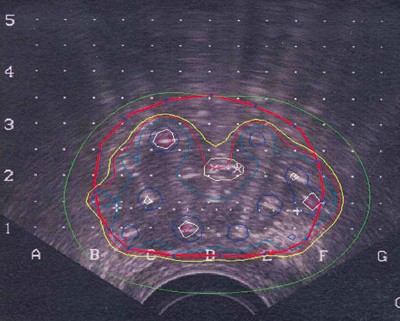
Figure 1 Isodose lines in the prostate representing different dose percentages through a slice of the gland. The thick red line shows the prostate capsule. The red line depicts the 100 % isodose and on the inside of this most tissue will receive at least 10 Gy
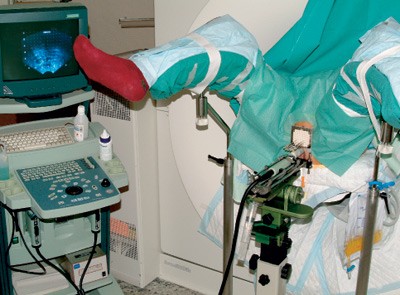
Figure 2 A patient in position for high-dose-rate brachytherapy: An ultrasound probe is placed in the rectum and a perforated plate with hollow steel needles is implanted through the perineum into the prostate (same coordinates as on the ultrasound screen). Every needle will be coupled to a channel with a connection to the treatment apparatus. After dose planning, a thread-shaped iridium source is inserted into each needle and a radiation dose tailored to each individual is given
The external beam radiation therapy is given to the prostate with photons from an external source. Four isocentric radiation fields are adjusted according to a CT-guided dose planning technique; one anterior, one posterior and two lateral. The treatment starts about 14 days after the last brachytherapy fraction, and 2 Gy is given five days per week until the total dose is achieved. Those who had two treatments with high-dose-rate brachytherapy received a total of 50 Gy with external beam radiation and patients with one brachytherapy fraction received 60 Gy.
Pilot study
A pilot study was planned with 15 - 20 patients at intermediary or high risk. We wished to assess the procedure’s probability of success and the toxicity during the first three to five months after treatment. The patients received one single high- dose fraction of 10 Gy followed by an external dose of 60 Gy given over six weeks. Treatment with two fractions is better in relation to radiation biology, but to ensure adequate treatment to all patients before the technique was well established, we wanted to use an external dose that was as high as possible.
Participation was voluntary. The patients received information both orally and in writing and they could withdraw from the study at any time. A regional ethics committee endorsed the study.
Observational study
After the pilot study was performed and evaluated as satisfactory, planning started for a prospective observation study with assessment of clinical and biochemical response, treatment related toxicity and quality of life (with internationally validated questionnaires). The patients received two 10 Gy fractions of high-dose-rate brachytherapy (with two weeks between the two fractions) followed by external beam radiation (50 Gy).
Patient inclusion
All patients with an intermediary or high-risk profile could be included in the pilot study; in the observation study one could also include low-risk patients with comorbidity and a contraindication for full dose external beam radiation. Examinations before treatment included TNM classification, volume calculations, bone scanning (scintigraphy), X-ray thorax and common blood samples, including PSA measurements.
The patients had local or locally advanced adenoma carcinoma in the prostate classified as T1c-T3A without regional lymph metastases (pN0, N0 in MRI) or distant metastases (M0), as assessed by bone scanning. The T-category was determined after palpation and cystoscopy by an urologist at the local hospital or at the Radium Hospital. All biopsies from the prostate and the removed lymph nodes were retrieved and reassessed at the Pathology department at The Radium Hospital, with reassessment of the Gleason score. The patients must have an expected lifetime of at least 10 years, be less than 75 years and in general good health, with an ECOG status of 0 - 1 (Eastern Cooperative Oncology Group: score from 0 to 4 for medical condition). Specific side effects from the brachytherapy procedure itself, and other side effects from both the brachytherapy and the external radiation treatment were recorded at the first control after three to five months, according to international toxicity criteria (10). Toxicity of grade 1 - 2 is usually mild and transient, toxicity of degree 3 - 4 is more bothersome and often requires treatment.
Patient exclusion
Patients with other malignant disease and those who had previously undergone radiation therapy towards the pelvis were excluded from the pilot study. Those with a high PSA value > 50 µg/L and those who were not suited for anaesthesia or minor surgery, for example bleeding disturbances, were also excluded. Unfortunate anatomical conditions for high-dose-rate brachytherapy, such as adipositas, narrow pelvis and adenoma nodules in the bladder neck also led to exclusion. Patients with a large prostate volume (> 60 cm³) that could not be included initially, could be included after three months of hormone treatment to reduce the gland size.
Endocrine therapy
All patients in the high-risk group were given additional androgen deprivation treatment. Patients with intermediary risk received the same hormone treatment if they had a PSA value > 20 µg/L, a Gleason score > 7 or a T3 tumour. Endocrine treatment is given for about three years, starting six months before radiation therapy. The treatment is given as depot injections with lutein-releasing hormone (LH-RH analogue) every 12th week. Antiandrogen (bicalutamide 50 mg × 1 orally) treatment should be given as prophylaxis against heat flashes and sweating (flare-up) for 30 days starting one week before the first injection.
Results
For three years from January 2004, 18 patients in the pilot study and 82 in the observational study received a total of 182 implantations. 88 of these patients were in the high-risk group and 11 in the intermediary group. One patient in a low risk group also received the high-dose-rate brachytherapy, as a full external beam dose was regarded as risky because of Crohn’s disease.
Initially, plastic needles were used, but after a needle fragmented in the prostate steel needles were used instead.
Neoadjuvant hormone therapy was given to 85 patients in the high-risk group (78 received treatment for six months and seven for three months) to reduce the size of the prostate. 22 patients had therefore not received endocrine treatment at the first control.
Median age for these 100 patients at the time of the first brachytherapy was 65 years (51 - 76 years). 62 % had T3A tumours, i.e. locally advanced disease with capsular penetration. The median PSA value was 16.5 µg/L (2.3 - 64.3 µg/L). 22 patients had aggressive disease; half of them (11) had a Gleason score of 8 and half a score of 9. All the included patients had been to their first control three to five months after the assessment of acute toxicity and PSA response. After a median follow-up time of eight months (4 - 40 months) from the first implantation 99 patients were biochemically free of recurrence, 81 had stabile PSA levels, 18 had falling PSA levels while the last patient had a slowly rising PSA level.
In two patients, high-dose-rate brachytherapy was not possible to do; adipositas was the cause in one of them, while the other had a large prostate with the lateral limitation of the gland behind bone tissue (pubic arch interference). Two additional patients that fulfilled the criteria were therefore included in the study, so the total number of assessed patients remained 100.
Results of the pilot and observational study
All the 18 patients in the pilot study who were asked to participate accepted inclusion in the observational study. 17 of these had a high-risk profile; the last had an intermediary risk.
Of 82 patients in the observational study, nine received only one fraction of brachytherapy combined with 60 Gy as an external radiation beam. There were different explanations for this- one patient could not have a planned external dose because of a high risk of normal tissue toxicity, one had bilateral hip replacements that gave artefacts in external CT images, two had a short-lasting urine retention after the first brachytherapy and in one biocompatible plastic needles had fragmented in the prostate. The others did not receive fraction number two because of capacity problems.
In the pilot study, specific side effects related to the implantation were temporary haematuria and short-lasting perineal pain for three patients and urinary retention of short duration in three patients.
In the observational study, four patients developed grade 3 toxicity in the urinary tracts. Two patients had urinary retention that was treated with a suprapubic catheter and transurethral resection of the prostate (TUR-P); one patient had dysuria and one had pollakisuria. In addition, one patient had grade 2 and six patients had grade 1 toxicity from the urinary tract.
Table 1 describes specific side effects. One can see that 72 patients (72 %) did not have any side effects after the intervention, irrespective of whether they had received one or two dose fractions. In those who received one fraction because of comorbidity, only five (55 %) were without side effects. Three months of follow-up showed that side effects from the urinary tract (toxicity grade 1 and 2) were the dominating acute effects of the combined treatment (tab 2). No patients had toxicity of grade 4. Rectal problems of grade 3 were only observed in one patient who developed diarrhoea. The treatment was associated with minimal bleeding.
|
Table 1 Specific side effects related to the brachytherapy procedure in 100 patients with prostate cancer
|
|
Dose brachytherapy
|
10 Gy × 1 Pilot study
|
10 Gy × 1
|
10 Gy × 2
|
Total
|
|
Number of patients
|
18
|
9
|
73
|
100
|
|
None
|
12 (66 %)
|
5 (55 %)
|
55 (75 %)
|
72 (72 %)
|
|
Haematuria
|
3
|
1
|
14
|
18 (18 %)
|
|
Haematospermia
|
0
|
1
|
0
|
1
|
|
Haematoma in the perineum
|
0
|
0
|
2
|
2
|
|
Perineal pain
|
1
|
0
|
0
|
1
|
|
Fragmentation of needle
|
0
|
1
|
0
|
1
|
|
Table 2 General side effects after brachytherapy combined with external beam radiation at the first control after 3 - 5 months
|
|
Dose brachytherapy
|
10 Gy × 1
|
|
10 Gy × 2
|
|
Number of patients
|
27
|
|
73
|
|
Grade of toxicity
|
1 - 2
|
3 - 4
|
|
1 - 2
|
3 - 4
|
|
Strong urge to urinate
|
8
|
0
|
|
18
|
0
|
|
Dysuria
|
4
|
0
|
|
5
|
1
|
|
Nocturia
|
5
|
0
|
|
5
|
0
|
|
Pollakisuria
|
8
|
0
|
|
9
|
1
|
|
Urinary incontinence
|
1
|
0
|
|
2
|
0
|
|
Bleeding from the rectum
|
0
|
0
|
|
3
|
0
|
|
Strong urge to defecate
|
5
|
0
|
|
7
|
0
|
|
Mucus per anum
|
0
|
0
|
|
8
|
0
|
|
Diarrhoea
|
0
|
0
|
|
0
|
1
|
Erection problems were captured consecutively with validated patient- based questionnaires and will be analysed after a longer observational period.
Discussion
High-dose-rate brachytherapy can be performed before or after external beam radiation or after half of the external beam radiation has been completed. We chose to give the brachytherapy before the radiation procedure. The procedure is resource-demanding and involves many professional groups: doctors, medical physicists, radiation therapists and nurses. This requires predictable preparations and start-up procedures. 72 % of our patients, who received high-dose-rate brachytherapy on common medical indications, had no specific side effects shortly after the interventions. If the 18 % who acquired temporary asymptomatic haematuria are included, 90 % of the patients were without physical discomfort after the implantations.
After high-dose-rate brachytherapy combined with external beam radiation, most of the acute side effects observed are related to the urinary tract. An explanation for this is probably the accurate treatment plan and fixation of the prostate in a certain distance from the rectum. Patients who receive full dose external beam radiation dose have most problems from the rectum.
In the follow-up period, a patient developed PSA elevation because of distant metastases. He probably had a tumour infiltration in the vesicular seminalis and should have been excluded from the study. The PSA level at the first control, after 3 - 5 months, cannot be used as a marker for treatment effect, as median follow-up time is far too short and 78 % of patients were receiving endocrine treatment.
However, in Kestin and collaborators’ comparative study, 67 % of patients who received this combined treatment were biochemically without recurrence after 5 years. For comparison, 44 % of those who received external beam therapy alone were without recurrence (11). In the first randomised phase 3 study, in which high-dose-rate brachytherapy combined with external beam therapy was compared with external beam radiation alone, it is shown that combination treatment gives a significantly increased disease-free survival (12). There is no difference in bladder toxicity, but those who were treated with high-dose-rate brachytherapy had significantly fewer acute side effects from the rectum.
In a patient group treated with conventional external radiation (dose 66 Gy) at our hospital, 5 % had lasting problems with urination and about 7 % developed long-term problems with proctitis (13). As shown by Pollack and collaborators (6), escalation of an external radiation dose from 70 Gy to 78 Gy will give many patients long-term side effects from the rectum and bladder in addition to an increased risk of impotence. Rectal toxicity increased significantly when the total dose increased. With the lowest dose, 12 % of patients had rectal side effects of grade 3 and 4, with the highest dose - 25 % had problems. The erectile function was intact or partly intact in 78 % after 70Gy and in 62 % of those who received 78 Gy.
At the Sahlgrenska hospital in Göteborg one noted that 7 % (on average) of patients developed urethral stricture three years after high-dose-rate brachytherapy (8). Since then, technology developments have enabled a more precise dosing in the prostate so overdosing to the urethra can be avoided. Publications describe improved cancer-specific survival and few long-term complications (8,11,14,15).
The purpose of fractioned radiation therapy is to save the normal tissue from late complications. Prostate cancer often has a slow growth rate that makes the tumour cells more sensitive to increased fraction dose. High-dose-rate brachytherapy combined with external radiation combines two different therapeutic radiation modalities with different side effect profiles. External beam radiation is given because of the risk of expanding extracapsular tumour; high-dose-rate brachytherapy in the form of dose escalation is given to increase local tumour control (boost). Experience has shown that adipositas and diabetes are conditions that increase toxicity with external beam treatment alone. Combination treatment should be considered in these cases, because the radiated area is then substantially reduced.
Clinical indications for high-dose-rate brachytherapy are listed in Box 2, contraindications are presented in Box 3.
Box 2
Special clinical indications for combined high-dose-rate brachytherapy and external beam radiation therapy irrespective of prognostic variables, when external therapy in full dose is difficult or contraindicated.
Inflammatory intestinal disease (ulcerative colitis, Crohn’s disease)
Symptomatic arteriosclerosis in the pelvis
Previous radiation towards the pelvis (for example in cancer testis)
Previous operation in the pelvis or lower abdomen
Replaced bilateral hip joint
When anatomical conditions rule out external beam radiation (not possible even with adequate shielding of the bladder and rectum)
Box 3
Contraindications to high-dose-rate brachytherapy
Obstructive urinating problems - IPSS > 15 (International Prostate Symptom Score)
Stenosis or stricture in the urethra
Tumour infiltration towards rectum, vesiculae seminales (T3B)
TUR-P < 6 months or large resection cavity
Large prostatic adenoma nodules in the bladder neck
Large prostate volume, i.e. > 60 cm³
PSA level > 50 - 60 µg/L
Rectum amputation
Conclusion
Curative radiation therapy of prostate cancer can be provided as high-dose-rate brachytherapy combined with external beam radiation. According to our first experience, there are few acute side effects of high-dose-rate brachytherapy, and contrary to external beam radiation alone - few side effects from the rectum. The indication is local or locally advanced prostate cancer without metastases in patients belonging to either an intermediary or high-risk prognostic group. High-dose-rate brachytherapy is also indicated in patients having an increased risk of unacceptable toxicity in the bladder and rectum with conventional external beam radiation doses. Treatment with high-dose-rate brachytherapy of patients with local or locally advanced disease is now a standard procedure at our hospital.