A majority of tuberculosis cases are caused by Mycobacterium tuberculosis, but in rare cases other bacteria belonging to the M tuberculosis complex could be the cause of disease. Pulmonary tuberculosis is often distinguished from extra.pulmonary tuberculosis. From an epidemiological viewpoint, it is most important to treat patients with pulmonary tuberculosis, as these cases can potentially transmit the bacteria to others. The treatment period for tuberculosis is long, at least six months, and incorrect treatment is a serious challenge both to the individual patient and to society as such. The prevalence of the disease has increased in many countries during the last decade, and more people are affected by the disease globally now than ever before (1). The main challenge in fighting tuberculosis globally is to ensure early detection and correct treatment of all infectious cases. Other challenges are the HIV epidemic and the increase of resistant tuberculosis.
Material and method
This review article is based on relevant literature retrieved from a non-systematic search in medical databases, on surveillance of the disease in Norway; and on current recommendations for prevention and control of tuberculosis in Norway and other countries.
Causes of resistant tuberculosis
Resistant tuberculosis is created by human beings. Populations of M tuberculosis, which have not been exposed to antibiotics against tuberculosis, contain certain resistant mutants. With incorrect treatment of tuberculosis, for example with monotherapy (one single drug) to which the bacteria is susceptible, the latter will be killed, but the non-susceptible bacteria will survive. The result can be a population of bacteria resistant to the drug used. The mechanisms of action and involved genes differ for the various drugs (tab 1). The World Health Organization now uses the terms resistance in «new versus previously treated patients» instead of «primary and acquired resistance» (box 1). Resistant tuberculosis transmits in the same way as common tuberculosis.
|
Table 1 Mutations resulting in resistance to first-line drugs
|
|
Drug
|
Important involved genes
|
Mechanism of action
|
|
Rifampicin
|
rpoB
|
Inhibits synthesis of nucleic acids
|
|
Isoniazid
|
katG, inhA, kasA
|
Inhibits synthesis of nucleic acids and other metabolic processes
|
|
Ethambutol
|
embB
|
Inhibits synthesis of cell walls
|
|
Pyrazinamid
|
pncA
|
Affects the cell membrane
|
Box 1
Types of resistance
Primary resistance is resistance demonstrated in samples from untreated patients.
Resistance in previously treated patients may be caused by development of resistance in the bacterial strain during treatment (acquired resistance), or by a new infection caused by a bacterium that is already resistant. Genetic characterisation with DNA fingerprint can determine whether or not a bacterial strain is identical to previous isolates from the same patient, and is needed to distinguish between these causes of resistance.
Normally, tuberculosis can be treated efficiently and at a reasonable cost with a standardized combination of four drugs referred to as first-line drugs: rifampicin, isoniazid, ethambutol and pyrazinamid. These drugs have been available since the 1950s (isoniazid and pyrazinamid) or the 1960s (rifampicin and ethambutol). The causes for development of resistance are complex: the doctor may have prescribed an inadequate combination of drugs or the patient may not have followed the doctor”s orders. Preventive treatment will not lead to development of resistance provided active disease is excluded. This is because the number of bacteria in a tuberculosis infection is extremely low. In rare cases the reason for developing resistance is poor absorption, or the fact that patients have to interrupt their treatment as a result of side effects. In societies where there is a breakdown of health services for various reasons, the possibilities of following up patients during treatment have been reduced. This has led to irregular access to drugs, drugs of poor quality and poor diagnostics and reporting systems (box 2). These conditions have been decisive for the serious tuberculosis situation a number of countries are struggling with at present.
Box 2
Most important causes of resistant tuberculosis and current preventive measures
Prescribed drug regimen was inadequate. Action: Proper training of medical staff
Patients have not been taking the drugs as prescribed. Action: Improved organization of treatment
Poor supply and/or poor quality of drug. Action: Ensure resources and quality standards for the drug supply
Uncontrolled access to drugs not included in the tuberculosis programs. Action: Strict control of the drug trade
Classification
Many drugs are affected by resistance, as mono-resistance or resistance in combination with other drugs (box 3) (1). Mono-resistance to streptomycin or isoniazid, or combined resistance to these two drugs is most common (tab 2). This also applies globally (2). Resistance to both rifampicin and isoniazid, the so-called multi-drug-resistant tuberculosis (MDR-TB) is of most clinical importance. Resistance to rifampicin is very frequently seen in combination with resistance to isoniazid, and is used as a marker for multi-resistant tuberculosis. A rapid test to determine resistance to rifampicin is therefore useful before start of treatment.
|
Table 2 Results from drug susceptibility testing of M tuberculosis isolates in Norway 2002 – 06
|
|
|
Reporting year
|
|
Resistance
|
Drug
|
2002
|
2003
|
2004
|
2005
|
2006
|
|
Monoresistance
|
Isoniazid (H)
|
3
|
8
|
10
|
9
|
7
|
|
Rifampicin (R)
|
|
2
|
1
|
|
|
|
Ethambutol (E)
|
2
|
|
|
2
|
1
|
|
Streptomycin (S)
|
8
|
21
|
19
|
20
|
14
|
|
Total number with mono resistance
|
|
13
|
31
|
30
|
31
|
22
|
|
Multiresistance
|
H+R
|
3
|
1
|
2
|
|
|
|
H+R+E
|
1
|
2
|
|
|
|
|
H+R+S
|
2
|
|
|
3
|
1
|
|
H+R+E+S
|
1
|
|
2
|
|
2
|
|
Total number with multi- resistance
|
|
7
|
3
|
4
|
3*
|
3
|
|
Poly-resistance
|
H+S
|
8
|
10
|
4
|
7
|
15
|
|
H+E
|
|
|
1
|
|
1
|
|
H+E+S
|
4
|
2
|
|
1
|
1
|
|
Total number with other combinations of resistance
|
|
12
|
12
|
5
|
8
|
17
|
|
Number of patients with results from drug susceptibility testing
|
|
192
|
273
|
246
|
214
|
225
|
|
Number (%) of patients with reported resistance
|
|
32 (17)
|
46 (17)
|
39 (16)
|
42 (20)
|
42 (19)
|
|
[i]
|
Box 3
Classification of resistance in tuberculosis
Mono-drug-resistance: Resistance to only one drug used for treatment of tuberculosis
Multi-drug-resistance (MDR-TB): Resistance to rifampicin and isoniazid, the two most important drugs in treatment of tuberculosis, and possibly also to other drugs
Poly-drug-resistance: Resistance to more than one drug, but without multi-drug-resistance
Extensive multi-drug-resistance (XDR-TB): Multi-drug-resistance and in addition detected resistance to one fluroquinolone and at least one of the three second-line injectable agents amikacin, capreomycin or kanamycin (1)
Treatment of resistant and multi-drug-resistant tuberculosis requires treatment with so-called second-line drugs. Such treatment is very expensive, requires a longer treatment period (two years with multi-drug-resistant tuberculosis) and often has side effects. Incorrect use of these drugs has also resulted in patients treated for multi-drug-resistant tuberculosis developing resistance to even more drugs. This is the reason for the growth of the so-called extensively multi-drug-resistant tuberculosis (XDR-TB) (3, 4). The treatment possibilities with drugs are therefore limited. Surgery can be an option if the disease is limited to certain parts of the lung.
Epidemiology
Resistant tuberculosis is a global problem on the increase. Global prevalence cannot be determined accurately because of variable quality of laboratories performing drug susceptibility testing and surveillance of drug resistance. According to reports from WHO and the International Union against Tuberculosis and Lung Disease, drug-resistant tuberculosis (including multi-drug-resistant tuberculosis) is found all over the world (2). WHO has estimated that 424 000 patients a year will be diagnosed with multi-drug-resistant tuberculosis, of whom 70 000 will be in the European region (5). The prevalence is especially high in countries under the former Soviet Union, including the Baltic countries, Kazakhstan, Uzbekistan and Russia, where multi-drug-resistant tuberculosis constitutes 10 – 26 % of cases (2, 3, 6, 7). However, WHO estimates that the number of patients with multi-drug-resistant tuberculosis is highest in China and India (3, 4). West and Central Europe and Africa reported the lowest level of multi-drug-resistant tuberculosis. The exception is South-Africa where resistant tuberculosis is prevalent, and where the outbreak of extended multi-drug resistant tuberculosis among HIV-infected patients is associated with an extremely high mortality (3, 4).
Results of drug susceptibility testing have been part of the surveillance of tuberculosis in Norway since 1978. Since then 57 patients have been reported with multi-drug-resistant tuberculosis of whom 7 (12 %) were born in Norway (fig 1). During the first 20 years, an average of one patient with multi-drug-resistant tuberculosis was recorded per year; this has increased to three per year during the last decade. 2 955 cases of tuberculosis were reported to the Norwegian Tuberculosis Registry during the period 1996 – 2006, of which 2 191 (74 %) were confirmed by culture. Among patients with culture confirmed tuberculosis, 39 were diagnosed with multi-drug-resistant tuberculosis (slightly fewer than 2 % per year). Of these, 32 patients (78 %) had pulmonary tuberculosis in the lungs, and 11 (28 %) informed they had previously been treated for tuberculosis. When including notifications up to October 2007, two additional patients have presented with multi-drug-resistant tuberculosis. Resistant tuberculosis in Norway is mainly found among patients born abroad who were infected by or had developed resistant tuberculosis before moving to Norway, and the information on previous tuberculosis and treatment history is often scarce. More recently, multi-drug-resistant tuberculosis in Norway has increasingly been found in people from the former Soviet Union, but none from north-western part of Russia which is closest to Norway.
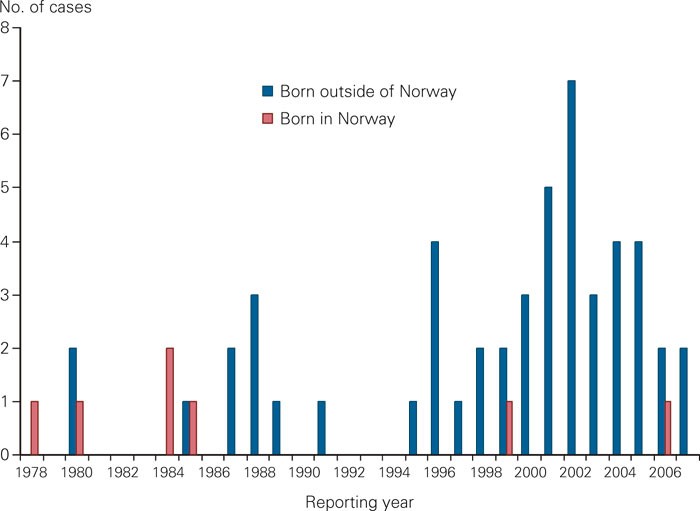
Figure 1 Reported cases with multi-drug-resistant tuberculosis in Norway from 1978 until October 2007 by origin (n = 57)
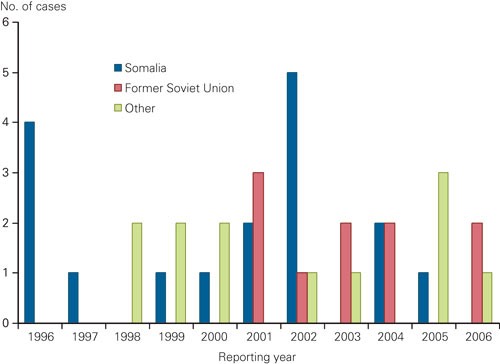
Figure 2 Reported cases with multi-drug-resistant tuberculosis in Norway from 1996 until 2006, by origin – Somalia, former Soviet Union or other (n = 39)
Despite the few cases of drug resistant tuberculosis, Norway has one of the largest documented outbreaks of multi-drug-resistant tuberculosis in Europe. The outbreak started in 1994 when a patient with an M tuberculosis strain, which in retrospect proved to be resistant to isoniazid, started treatment with only three drugs. The patient had a complicated life and was not treated under direct observation. In addition, he did not meet for follow-up and defaulted from treatment (8). After one year the patient was re-admitted to hospital with infectious tuberculosis and by then it was evident that the same bacterial strain had become resistant also to rifampicin. In the course of the next six years identical bacterial strains were found in 24 patients. Advanced laboratory-based and epidemiologic comparisons indicate that this case was the index of the outbreak; the story illustrates the serious consequences if one single patient is lost to follow-up during the treatment (9, 10).
Diagnostics
The diagnosis of tuberculosis is confirmed by culturing of M. tuberculosis. It is of outmost importance to have representative samples collected and sent for culturing before starting a preliminary treatment regimen. Direct microscopy of sputum is necessary to assess the degree of infectiousness. Polymerase chain reaction (PCR) directly on the clinical sample from the respiratory tract can provide a rapid tentative diagnosis by showing the presence of M. tuberculosis-complex, thus excluding other types of mycobacteria.
Liquid media (MGIT-tubes or Bact./Alert-bottles) is used by all relevant Norwegian laboratories for culturing of mycobacteria, while some use solid medium (Löwenstain-Jensen) in addition. In liquid media growth is apparent after 1 – 2 weeks, whilst growth on solid media takes somewhat longer. With low bacterial load cultivation can take 4 – 6 weeks, which explains why it takes time before confirmation of the tuberculosis diagnosis and drug susceptibility results are ready.
A few Norwegian laboratories perform tests for species identification and resistance against first-line drugs. All M tuberculosis isolates in Norway are sent to the reference laboratory for mycobacteria at the Norwegian Institute of Public Health, which tests or confirms the identification, examines susceptibility and performs molecular epidemiological analyses (box 4). If resistance to first-line drug(s) is found, further susceptibility testing for second-line and possibly third-line drugs (box 5) is performed there. Testing of second and third-line drugs is especially challenging as the test methods for some drugs are not very standardized, and little documentation is available on the association between laboratory results and the clinical treatment effect (3, 11). The Norwegian Institute of Public Health is also part of an international system for quality control of drug susceptibility testing.
Box 4
Microbial diagnosis of tuberculosis in Norway
Detection of M tuberculosis:
Direct smear microscopy: Detection of acid-fast bacilli
PCR: Detection of the M tuberculosis-complex
Culture: Solid media; Löwenstein-Jensen (most often) liquid media; MGIT 960, Bact/Alert (=MB/Bact)
Identification of mycobacteria
PCR on clinical sample: M tuberculosis-complex
Morphology, macro- and microscopic
DNA hybridisation
Biochemical tests
Genotyping
16S rRNA-sequencing
Drug susceptibility testing of M tuberculosis
Phenotypic resistance in liquid media (MGIT 960, Bactec 460)
Genotypic resistance, rapid test for mutations in the rpoB-gene (rifampicin) and possibly the katG-gene (isoniazid)
Molecular epidemiological methods
-
DNA fingerprinting
Restriction fragment length polymorphism (RFLP)
Spoligotyping
Box 5
Drug susceptibility testing, diagnostic routines in Norway
-
Genotype resistance (all isolates)
Phenotype resistance
-
Isolates
are tested against first-line drugs: isoniazid (H), rifampicin (R), ethambutol (E), pyrazinamid (P) and streptomycin (S)
If resistance is detected to one or several of the first-line drugs (with the exception of mono- resistance to streptomycin), extended routine testing is centralized and carried out at the Norwegian Institute of Public Health.
With confirmed resistance which does not include rifampicin, testing is carried out with the following drugs: amikacin, ofloxacin, clarithromycin, clofazimin, rifabutin, p-aminosalicylic acid (PAS).
If resistance to rifampicin is detected (indicating multi-drug-resistance), the following drugs are also tested: ethionamid, capreomycin, thiacetazon, kanamycin, linezolide, amoksicillin/clavulanat
Treatment
The first cases of drug resistant tuberculosis were discovered as early as in the 1950s after the introduction of streptomycin as mono-therapy. Therefore, tuberculosis must always be treated with standardized combinations of various drugs. Due to the slow growth of the bacteria and the low potential for mutations, the likelihood of developing resistance in connection with combined treatment, is limited. Tuberculosis sensitive to all first-line drugs, is treated with a standardized treatment regimen for six months. Choice of treatment regimen for resistant tuberculosis depends on the drug susceptibility results. Generally, the treatment regimen will contain as many first-line drugs as possible followed by second-line and possibly third-line drugs chosen according to the drug susceptibility pattern.
As it can take several weeks to obtain the drug susceptibility result, many patients have already started the treatment with four drugs” standard regimen before test results are available. If the isolate shows sensitivity to all first-line drugs, ethambutol can be considered discontinued. If drug resistance is detected, interruption of the tuberculosis treatment can be considered depending on clinical criteria, until additional drug susceptibility results are available and a tailored treatment can be prescribed. Alternatively, a preliminary treatment regimen based on empirical considerations can be prescribed until complete drug susceptibility results are available.
If the doctor suspects resistant tuberculosis at the time of diagnosis either because of the patients medical history, known exposure to a case with resistant tuberculosis or the patient originates from a country with a high incidence of resistant tuberculosis, it may be considered to postpone start of treatment until drug susceptibility results are available, at least for rifampicin and isoniazid.
Globally, the cure rate for patients treated for multi-drug-resistant tuberculosis is lower than for patients treated for tuberculosis sensitive to first-line drugs (1). Most of the patients with multi-drug-resistant tuberculosis in Norway receive adequate treatment as we have favourable routines for drug susceptibility testing, high quality diagnostic services and access to all types of drugs. Monitoring of treatment outcome for patients with multi-drug-resistant tuberculosis in Norway from 1996 until 2006, show that 67 % of patients are currently receiving correct treatment or have completed such treatment. The most important cause of discontinuation of treatment is loss to follow-up. This is very unfortunate and can constitute a serious threat to public health. Treatment options for drug resistant tuberculosis are already reduced and additional resistance will limit the treatment possibilities even more. It is adamant to establish a good collaboration with the patient as early as possible. As the treatment takes so long, and the drugs partly have serious side effects (3, 12), the patient faces multiple challenges in practical, medical and emotional terms. The reasons for tuberculosis patients abandoning their treatment can comprise language problems, cultural differences, poor communication between various officials and levels in the health service, drug abuse, and lack of residence permit, a place to live or work (13). Collaboration between the Norwegian Directorate of Immigration and the Immigration Appeals Board ensures that patients applying for political asylum during an ongoing tuberculosis treatment are granted a temporary residence permit until the treatment is complete.
Prevention and control
There are general international agreements on how tuberculosis, including multi-drug-resistant tuberculosis should be prevented, diagnosed and treated (1, 14 – 16). The most important preventive measure in the control of tuberculosis is timely detection and correct treatment whether the patient is ill with a resistant or sensitive bacterial strain. This will ensure the recovery of the individual patient, prevent further transmission in society, and prevent development of resistant or further resistant bacteria. Based on experience in other countries, we should be especially vigilant towards outbreaks in drug abuse environments. Contact investigations following exposure to multi-drug resistant tuberculosis are the same as for tuberculosis in general. At present, however, there are no international suggestions as to which drugs should be used for preventive treatment. The objective of follow-up of contacts is early diagnosis in case of disease. Special measures to prevent development of resistance are closely linked to the organization of treatment.
Organization of treatment
Treatment of tuberculosis is subject to strict regulations in Norway. Infection specialists, lung specialists or paediatricians are responsible for starting and choosing the correct regimen for all tuberculosis treatment. In addition, the treatment of multi-drug-resistant tuberculosis is centralized to hospitals chosen by the regional health trusts, cf. Regulation on tuberculosis control § 3-3 (17). The grounds for such centralization is the scarcity of patients in Norway, the complex treatment and the fact that competence can only be acquired and maintained in a small number of places. The Norwegian Institute of Public Health has established a group of specialists for multi-drug-resistant tuberculosis in order to ensure the building of competence and exchange of experience among the specialists who are responsible for such treatment (18). According to the Regulation on tuberculosis control § 3-3, all treatment of tuberculosis in Norway must take place under direct observation (17). This requires that health personnel or other instructed personnel observe that patients take their medication as prescribed by the doctor. This applies to all the patients during the entire treatment period. This was determined so it should be virtually impossible to develop resistance during treatment in Norway. To ensure that directly observed treatment is carried out in a patient-friendly manner, the tuberculosis coordinator is responsible for establishing an individual treatment plan for all patients. Directly observed treatment is especially important in connection with multi-drug-resistant tuberculosis, as even slight deviations from the drug intake can result in resistance to more drugs.
The future in Norway and globally
The future tuberculosis situation in Norway will be influenced by tuberculosis within and beyond Norway’s borders. The situation in the former Soviet Union and in Africa south of Sahara is especially worrying. Areas with a high prevalence of HIV will, to a larger extent than now, overlap with areas where multi-drug-resistant tuberculosis is prevalent. In some countries, 80 % of tuberculosis patients are HIV-positive, and an increasing number of these will have access to HIV treatment. A coordinated effort against the two diseases is necessary (19). With early diagnosis and correct treatment, there is no increased risk for developing multi-drug-resistant tuberculosis among patients who are also HIV-positive, but drug interactions in connection with simultaneous antiretroviral treatment are a challenge. An increasing number of countries have access to drugs for treatment of multi-drug-resistant tuberculosis through international partners such as the WHO’s Green Light Committee (GLC). GLC ensures access to second-line drugs at heavily subsidized prices, while the Global Drug Facility (GDF) makes procurement (and financing for 1.line drugs) and the Global fund for aids, malaria and tuberculosis (GFATM) also covers 2.line drugs. The condition for receiving help is that patients undergoing treatment are followed up in accordance with international recommendations, thus preventing the development of even more resistance.