Brugada syndrome was first described in 1992, and is characterized by atypical right bundle branch block with persistent ST-segment elevation in right precordial leads in the electrocardiogram (ECG) in the absence of a structural heart disease (1). Initially it was assumed to be a very rare condition, but over time the syndrome has been discovered in all parts of the world. It seems to be especially prevalent in South East Asia. The condition is perceived as an important cause of sudden cardiac death in young people who are otherwise healthy (2). It is therefore of great importance that the medical community is familiar with this syndrome.
Material and methods
The diagnostic approach and treatment of the Brugada syndrome is discussed within the context of a case presentation supported by a non-systematic review of literature retrieved from PubMed.
A 51-year-old man was hospitalized after an accident. ECG revealed ST- segment elevation in leads V1-V3 and a right bundle branch block. The patient’s regular general practitioner (GP) was advised to check the ECG. In case of persisting abnormalities, the patient should be referred to a cardiologist in order to exclude any type of structural changes of the right ventricle. The patients GP sent us the ECG (fig 1a). It turned out to be nearly identical with an ECG recorded 17 years earlier (fig 1b), when the patient was hospitalised with pulmonary embolism. This time we suspected Brugada syndrome and the patient was examined further. A newly recorded ECG (fig 2) still showed a typical pattern in the right precordial leads. Holter registration showed a sinus rhythm with few ventricular premature beats, some couplets and some episodes with three ventricular premature beats in series (triplets). A stress ECG was without pathological findings except for some ventricular premature beats after stress (none during stress). Echocardiography was normal and there were no signs of pathological abnormalities of the right ventricle. MRI of the heart (functional examination and injection of contrast medium to rule out delayed enhancement) was within normal limits. A blood sample, sent to the medical genetic laboratory at Rikshospitalet, showed that the patient was heterozygous for the splice site mutation 3991-1, G > C within intron 21 of the SCN5A gene. This mutation affects RNA splicing and leads to an abnormal protein.
The patient was referred to St. Olavs Hospital for electrophysiological examination and an implantable cardioverter-defibrillator (ICD) was considered.
Electrophysiological examination revealed a prolongation of atrial-His time, but otherwise normal basal conditions. Ventricular fibrillation was induced through programmed stimulation of the right ventricle (eight impulses in 400 ms intervals followed by two impulses after 240 ms and 200 ms). The patient lost consciousness, but was electro- converted without complications. ECG in the initial situation and after applying 150 mg flecainide intravenously showed first degree AV block, a right bundle branch block pattern, and a saddle-shaped ST-segment elevation of 1 – 3 mm in V1 – V3 (type 2), which is not diagnostic for Brugada syndrome. ECG with V1 – 3 electrodes adjusted one intercostal space in the cranial direction, showed abnormalities (type 1) typical of Brugada syndrome. The signal-averaged ECG was clearly abnormal. Coronary angiography was normal.
Family history revealed no evidence of sudden death or frequent loss of consciousness in close relatives. The patient specified two episodes of nausea, both after standing up, which were classified as orthostatic. He had never fainted, never noticed tachycardia or woken abruptly during sleep. His heart condition was clearly asymptomatic, and after discussion in the group of electrophysiologists it was concluded that there was no indication for implanting a cardioverter-defibrillator at the time. This conclusion was considered to be in line with international guidelines (3). The patient was advised to contact a doctor immediately in case of consciousness disturbances, and to take medication for lowering body temperature in case of fever. He should otherwise live a normal life. A check-up by the cardiologist was scheduled in a year’s time.
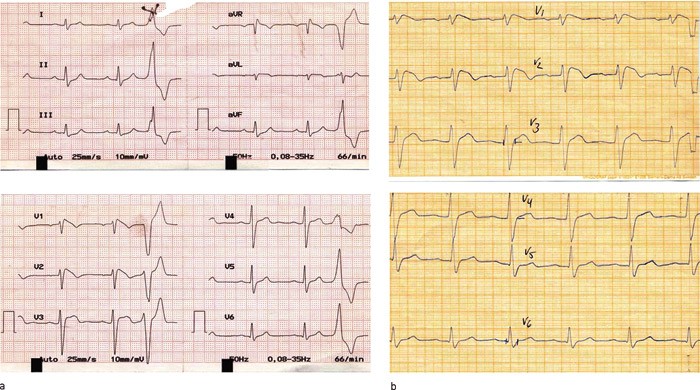
Figure 1 ECG (25mm/s) recorded by the GP at follow-up (a) showed abnormalities of the ECG (50mm/s) almost identical with those recorded for the patient in connection with a hospitalisation 17 years earlier (b). PQ-time is prolonged, ST-segment is elevated, and the QRS-complex expanded. The pattern corresponds to type 1-ECG in Brugada syndrome
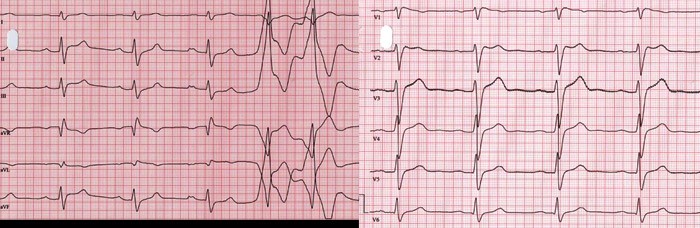
Figure 2 ECG (50mm/s) recorded during the most recent examination of the patient. The pattern corresponds to type 2-ECG in Brugada syndrome. In addition to repolarisation abnormalities, prolongation of P-wave, and of PQ and QRS-intervals can also be seen
Epidemiology
The prevalence of Brugada syndrome is estimated to be five in 10 000. Apart from accidents, the syndrome is one of the most prevalent causes of death amongst men under 40 years of age, especially in countries in which it is endemic (2). The condition is assumed to be far less common in Europe and the USA. As the typical ECG abnormalities may be intermittent, it is difficult to estimate the correct prevalence of the disorder. Despite autosomal dominant inheritance, clinical manifestations are eight times more prevalent in men than women (4). The cause of this sex difference is unknown.
Diagnostic procedures
Three different repolarisation patterns can be distinguished in the right precordial ECG leads. Type 1 is diagnostic for Brugada syndrome. In this case, the ECG shows ST-elevation of a least 2 mm with a typical coved shape in at least two of the three right precordial leads (V1 – V3), followed by a negative T-wave (fig 1a and 1b). In addition, there is a complete or incomplete right bundle branch block. The diagnosis Brugada syndrome is definite when the ECG shows a type 1 ST-abnormality and when one of the following criteria is met: documented ventricular fibrillation (VF), polymorphic ventricular tachycardia (VT), family history of sudden cardiac death under age of 45, family members with type 1 ECG abnormalities, inducible VT by electrical stimulation, syncope, or sleep apnea. There is a clinically important distinction between Brugada syndrome with ECG abnormalities including one of the additional criteria, and patients with Brugada-type ECG patterns without any of the additional criteria. The latter are not diagnosed with Brugada syndrome.
For cases in which the ECG manifestations of Brugada syndrome are concealed, they may be unmasked by sodium channel blockers i.v. (e.g. flecainide 2 mg/kg, up to a maximum of 150 mg). However, it is important to realise that the sensitivity of this test is unknown, but that it is definitely less than 100 % (4). One study showed a sensitivity of 77 % in a subgroup of patients who were carriers of a mutation in the SCN5A gene (5). Asymptomatic patients with an ECG type 1 pattern are usually not subjected to the provocation test, as this provides little additional information. The typical Brugada ECG pattern can also appear under the influence of external factors, such as an overdose of tricyclic antidepressants, fever, cocaine, anaesthetics etc. (6). It is unclear whether these patients are at higher or lower risk of sudden death.
Type 2 ST-segment elevation has a saddleback appearance with ≥ 2 mm elevation at the highest and ≥ 1 mm at the lowest point, and either a positive or a biphasic T-wave. Type 3 shows either a saddleback or upwardly bent ST-segment elevation at < 1 mm (fig 3) (4). All three distinct ECG patterns can be observed in the same patient if ECG is recorded at different points in time, as was the case with our patient (fig 1 and 2). This is an important point to be aware of, as ECG patterns of type 2 and 3 can easily be classified as unspecific and the correct diagnosis may thus be missed.
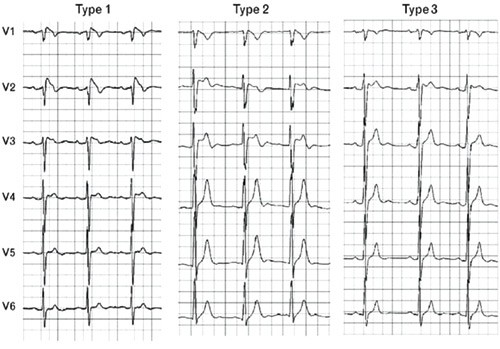
Figure 3 Examples (25mm/s) of the three different ECG morphology types typical of the Brugada syndrome (4)
It is important to realise that a number of other conditions can lead to ECG abnormalities similar to those in the Brugada syndrome (2, 7) and that such ECG abnormalities can appear after cardioversion (8). It should be noted that ST- elevation in the right precordial leads may also be observed with well-trained athletes (fig 4), but these differ from those in the Brugada syndrome as the ST-segment is ascending or horizontal, and is not influenced by sodium channel blockers (2).
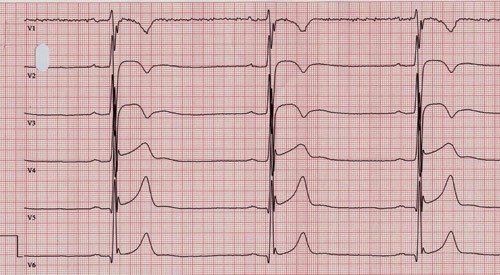
Figure 4 ECG (50mm/s) with precordial leads recorded from an athlete specialising in long-distance running
Depolarisation disturbances such as prolonged P-wave, PR and QRS intervals are often seen, especially in Brugada patients with the sodium channel (SNC5A) mutation (9). Our patient also had prolongation of these intervals (fig 2).
Genetics
Inheritance is autosomal dominant. The first and so far only gene clearly associated with Brugada syndrome is SCN5A, which codes for the cardiac sodium channel (10). Mutations in SCN5A reduce access to sodium ions, disturb the ion balance of the action potential, and form the basis for the cardiac rhythm disorders manifesting as ventricular tachycardia and ventricular fibrillation (11). SNC5A-mutations have been detected in around 25 % of patients, which indicates that mutations in other genes may also cause the condition (12).
Risk stratification and treatment
Brugada syndrome often presents with syncope and sudden death. This typically occurs during rest or sleep, but potentially fatal cardiac arrhythmias may also be triggered by fever, sizeable meals and significant alcohol intake (13, 14). The only effective treatment for prevention of sudden death is implantation of a cardioverter-defibrillator. The challenge for clinicians is to identify patients who are likely to benefit from implantation of a defibrillator for prophylactic purposes. Risk stratification for identification of patients with a risk of sudden death has been one of the aims of Brugada syndrome research (15, 16). Studies have shown that patients with spontaneous pathological ECG and a history of syncope are at the highest risk (17). Implantation of a cardioverter-defibrillator has proven to be an effective primary prophylactic measure in these patients (18).
The usefulness of several non-invasive parameters has been assessed for risk stratification in Brugada syndrome. Most of these parameters were shown to lack sufficient positive predictive value for identifying candidates who would benefit from cardioverter-defibrillator implantation. A relatively recent study indicates that a combination of late potentials and spontaneous abnormalities of the ST-segment in ECG was associated with a high risk of subsequent events (19). However, new and larger studies are required before these findings can be implemented into clinical practice.
Even more tenuous is the role of programmed electrical stimulation for identifying high-risk patients. The results have been divergent and literature provides no evidence that this type of invasive examination plays a role in risk stratification of Brugada syndrome (20). American and European guidelines suggest that programmed electric stimulation is not applicable for risk stratification in Brugada syndrome (3).
Opinions differ regarding usefulness of cardioverter- defibrillator devices in asymptotic patients (3). As the benefits are poorly documented, it is advisable to be conservative and in any case to include the patient in the decision-making process.
Patients with documented ventricular tachycardia that has not resulted in heart failure should be considered for implantation (3).
Pharmacological treatment has not been proven to be effective in Brugada syndrome (4). Beta-blockers and amiodarone have not shown any clear effect (1). Several other drugs have been tested, and it has been reported that quinidine may lead to a normalisation of the ST-segment in Brugada syndrome patients (21). However, few clinical studies have examined the effectiveness of quinidine (22). More well-designed clinical studies are required to establish the effects of various drugs.
General recommendations
Several drugs have been reported to enhance ST-segment elevation in Brugada syndrome. This concerns antiarrhythmic agents (class Ia and class Ic), beta-blockers, tricyclic antidepressants, local anaesthesia, propofol, lithium, cocaine and others (4). Clearly, these drugs should be avoided.
Fever has been shown to trigger fatal events and it is therefore recommended to reduce fever with medication in all patients with Brugada syndrome.
In accordance with published guidelines, it is recommended to refrain from athletic activities (23).
Brugada syndrome constitutes an important differential diagnosis in case of syncope. The only available treatment is implantation of a cardioverter- defibrillator, which can be lifesaving. It is therefore essential that physicians who examine patients with episodes of syncope are familiar with Brugada syndrome.