Lumbar puncture, also known as spinal puncture, involves passing a needle through the wall of the dural sac (Fig. 1) (1) and into the subarachnoid space, which is filled with cerebrospinal fluid, in the lumbar portion of the back. This is normally done in connection with diagnostics – to measure the pressure in the subarachnoid space, to analyse cerebrospinal fluid, to inject contrast medium for myelography or in connection with spinal anaesthesia. Lumbar puncture may occasionally have a therapeutic purpose, for example in cases of idiopathic intracranial hypertension. The dural sac may also have a hole after epidural anaesthesia/analgesia.
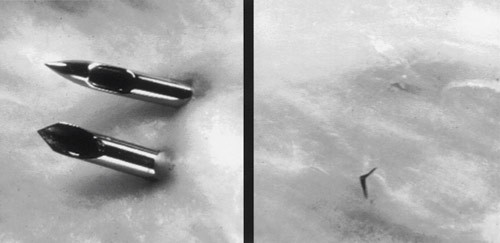
Figure 1 Hole in dural sac caused by pencil point needle (conical, atraumatic needle) and Quincke ground needle (cutting). Reproduced with permission from Neurology (1)
Lumbar puncture may cause post-dural puncture headache (PDPH). The «father of spinal anaesthesia», August Bier (1861?– 1949), is believed to have reported the first cases well over 100 years ago (2). The purpose of this article is to provide an updated status for the condition.
Method
The article is based on a discretionary selection of articles that we found by searching in PubMed on the following search phrases: «headache after lumbar puncture», «post-dural puncture headache», «post-lumbar puncture headache», «post-puncture headache», «post-spinal headache», «epidural blood patch». The search was concluded on 31 August 2011. Only English-language and Scandinavian literature were considered. Weight was attached to the results of randomised controlled studies, consensus documents and systematic overviews.
Definition
According to the International Classification of Headache Disorders (ICHD-II), post-dural puncture headache is iatrogenically conditioned orthostatic headache caused by low pressure in the spinal fluid space. Diagnostic criteria are listed in Box 1 (3). It is worth noting that these headaches can occur considerably later than five days after a lumbar puncture, and that at worst the condition may last for months and even years (4).
Box 1
Post-dural puncture headache (according to ICHD-II)
The headache occurs within five days of a lumbar puncture
The headache intensifies within 15 minutes of the patient sitting or standing up from a recumbent position, and abates within 15 minutes of the patient lying down
-
The headache is accompanied by at least one of the following:
Stiff neck
Tinnitus
Hyperacusis
Photophobia
Nausea
The headache disappears spontaneously within a week or within 48 hours after the cerebrospinal fluid leak has been effectively treated (normally with an epidural blood patch)
Incidence
The risk of developing a headache as a result of a lumbar puncture depends on a number of factors, and the incidence will therefore vary widely, depending on the populations studied and the needles and techniques that have been used (5, 6). The diameter, or gauge (gg), of the lumbar puncture needle (7), and the shape of the point (Fig. 1) appear to be the most important individual factors with a bearing on the incidence (5, 6, 8 – 12).
In a review of literature from 1966 to 2000, Evans et al. found that when needles of gauge 20 – 22 gg were used (typical of diagnostic spinal puncture) the incidence was 20 – 40 % (5). Post-dural puncture headache occurs roughly twice as often with diagnostic lumbar puncture as with spinal anaesthesia. The main reason is almost certainly that a thin needle, often with an atraumatic point (11), is usually used in spinal anaesthesia.
In 2001, Strupp et al. showed that over 12 % of 115 patients who were subjected to diagnostic lumbar puncture with a 22 gg (0.7 mm) atraumatic needle suffered post-dural puncture headache, while over 24 % of 115 who were given a lumbar puncture with a 22 gg traumatic needle suffered a headache (1). This finding provided the basis for an American recommendation to use a 22 gg atraumatic needle for diagnostic lumbar puncture (9). A later study with 58 patients has shown an even greater difference (36 % versus 3 % post-dural puncture headache) when an atraumatic needle is used (10).
International practice (11, 12) and practice at most neurological departments in Norway is not in line with these findings or with the American recommendation. An informal inquiry to all eighteen heads of neurology departments in Norway in 2011 showed that only three departments routinely used an atraumatic needle for diagnostic lumbar puncture (unpublished data).
Post-dural headache may also occur in connection with unintentional dural perforation, for example in unsuccessful attempts to insert an epidural catheter in obstetric patients, but this is relatively rare (in about 1 %) (7). It is nonetheless worth noting that the majority of birthing mothers suffering a perforated dura develop post-dural puncture headache.
Pathophysiology
As a result of buoyancy in the cerebrospinal fluid, the weight of the structures in the central nervous system is reduced to around 50 g (13). The pressures and tensions on the central nervous structures are accordingly reduced to a minimum. When the patient is in a vertical position, the pressure in the cerebrospinal fluid is negative, at around –10 mm Hg (14), but this increases markedly when the patient is in a horizontal position, usually to around 7 – 15 mm Hg (15). An older explanatory model (16) for headache caused by spinal puncture is that the pressure is too low as a result of persistent spinal fluid leakage through the hole in the dura, causing traction on pain-sensitive structures (meningeal membranes, blood vessels and nerves) (17 – 19).
The underlying mechanisms are undoubtedly more complex (20 – 25). According to the Monro-Kellie-Burrows doctrine (the sum of the volumes of the cerebrospinal fluid, the blood and the brain tissue in the skull remain constant), loss of cerebrospinal fluid may result in compensatory intracranial vasodilation. Relative cerebrospinal fluid hypovolaemia (23) which results in painful, possibly adenosine-receptor-mediated (24), vasodilation is thus another main hypothesis. In a clinical trial, Clark et al. found that a low level of substance P, a neuropeptide associated with neurogenic inflammation, resulted in a three times higher risk of developing headache after spinal puncture (25). As a result, it was postulated that those who were predisposed were hypersensitive to substance P as a result of premorbid upregulation of its receptor (neurokinin-1 receptor).
Risk factors
The risk factors for headache after spinal puncture can be classified as non-modifiable or modifiable (Table 1) (6, 25). The incidence is relatively low in children (26) and inversely proportional to age in adults, with the highest incidence in the age group 20 – 40. Post-dural puncture headache seldom occurs in persons aged over 60 (5, 6, 27). Women are twice as much at risk as men (5, 6, 27). Persons with migraine or other chronic headache, and those who have previously had post-dural puncture headache, have about three times as high a risk of developing the condition (5, 22). In a study with 501 patients, Kuntz et al. (28) found that a low body mass index (BMI) was a risk factor for headache after spinal puncture. The difference in average body mass was very modest, however (24.3 kg/m² versus 25.8 kg/m²), and the clinical relevance of this finding is uncertain.
|
Table 1 Risk factors for developing post-dural puncture headache. Modified after Bezov et al. (6)
|
|
Non-modifiable
|
Modifiable
|
|
Age
|
Size and type of needle
|
|
Gender female
|
Technical construction
|
|
Low body mass index?Known headache problems or previous post-dural puncture headacheLow concentration of substance P in cerebrospinal fluid (25)
|
Angle of needle and the cutting surface of the needle on puncturing Replacement of mandrin in the needleExperience (of epidural/spinal anaesthesia)
|
As previously mentioned, the most important modifiable risk factor is the needle used in the procedure. The calibre of the needle is directly associated with the incidence of post-dural puncture headache (5, 29, 30). The larger the needle, the larger the perforation in the dura, and the higher the risk of a persistent cerebrospinal fluid leak. However, needles that are too thin cannot be used, for practical reasons. If, for example, a needle of < 22 gg were to be used for diagnostic lumbar puncture, collecting cerebrospinal fluid would take an unreasonably long time. The American Academy of Neurology (AAN) therefore recommends that 22 gg be the thinnest needle used for diagnostic lumbar puncture (9).
As previously mentioned, using an atraumatic needle can also substantially reduce the incidence of post-dural puncture headache (1, 5, 10, 31). A non-cutting needle makes a smaller puncture hole in the dura than a cutting one, because the dural fibres are pushed aside instead of being cut. With an atraumatic needle, an «introducer needle» is usually used initially to penetrate the skin, and for this reason the use of atraumatic needles tends to be regarded as technically more challenging. This may be one reason why traumatic needles are still used to a large extent in neurological circles. However, studies indicate that a cutting needle may be the most difficult to use (30).
A higher number of punctures, due to inexperience on the part of those performing the lumbar puncture, may increase the incidence of post-dural puncture headache somewhat (32), but as a rule these technical difficulties are of minor importance. Nor do the patients’ posture, degree of hydration, puncture level, opening pressure, quantity of cerebrospinal fluid drawn or their remaining lying in bed after the lumbar puncture appear to have a major bearing on the development of post-dural puncture headache (5, 33).
In a meta-analysis of 2006, Richman et al. showed that the angling of the needle during penetration, cranially rather than perpendicular to the long axis of the spine, may significantly reduce the incidence of post-dural puncture headache (34). Replacing the mandrin in the needle before the needle is removed has also proved to be beneficial, particularly when atraumatic needles are used (5).
Clinical characteristics and diagnostics
Nine out of ten patients with post-dural puncture headache develop symptoms within 72 hours of a lumbar puncture (3, 27). The headache occurs or is exacerbated when the patient is upright, and abates or goes away when the patient is lying down. The exacerbation after the patient stands up normally occurs within 20 seconds, as does the relief when the patient lies down (15). The location, quality and intensity of the pain are of little diagnostic value.
The headache may incapacitate patients (35), and quite often leads to hospitalisation (36). Pulsating pain accompanied by nausea and hypersensitivity to light and sound can make it difficult to distinguish the condition from a migraine attack. As indicated by the diagnostic criteria (Box 1), loss of hearing and tinnitus are not unusual. When the headache is not postural, accompanied by cranial nerve effects, stiff neck, fever or visual impairment, it is important to exclude other conditions. Intracranial venous thrombosis, haemorrhaging, meningitis and preeclampsia are important differential diagnoses (5, 37).
In cases of low-pressure headache, including post-dural puncture headache, MRI of the head tends to show diffuse pachymeningeal enhancement due to the contrast medium (Fig. 2) and reduced ventricle size; the cerebellar tonsils may extend down into the foramen magnum and basal cisterns may be flattened or eliminated, or the hypophysis may be enlarged (11). In the event of doubt, CT myelography, cisternography or spinal MR with thin sections may reveal where the cerebrospinal fluid leak is located (38).
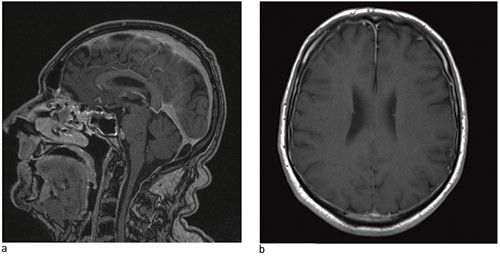
Figure 2 Cerebral MRI – a) from the side, b) from below – shows pachymeningeal enhancement with gadolinium in a man with postural headache and lumbar spinal fluid leakage. © Nordland Hospital Bodø
Treatment and prognosis
Post-dural puncture headache has in principle a self-limiting course. Given a conservative approach in the form of rest, good hydration and treatment of symptoms, over 50 % of patients recover within four days, just over 70 % within a week and over 85 % within six weeks (27, 39). Caffeine is first-line treatment, and a recently published Cochrane report documents symptom relief and a shorter course of illness where caffeine is used (40). Gabapentin, theophylline and hydrocortisone can also alleviate the headache, but not shorten the course of the illness (40).
Blood proves to coagulate on contact with cerebrospinal fluid (41), and the reason for applying an epidural blood patch is that the blood will seal the dural hole created by the puncture needle. The procedure consists of extracting 10 – 30 ml of blood (6) from one of the patient’s veins and injecting it slowly into the epidural space. After the procedure, the patient should remain lying down for 1 – 2 hours. Over 75 % of patients will be cured of their headache after this. If the attempt fails, a better result can often be obtained by repeating it (7, 35, 42). Some of the effect may be due to spontaneous remission, since the result is poor if the treatment is administered prophylactically (42) or within 24 hours of the lumbar puncture (43).
CT-guided injection of fibrin glue can be effective if application of an epidural blood patch fails (44). Surgical closure of the leak, which in the event must be located radiologically, may occasionally be a last resort (45). Complications rarely occur with epidural blood patches, but adhesive arachnoiditis, subdural haematoma and bacterial meningitis have been reported (46). There is no consensus as to when an epidural blood patch should be offered, but it does not seem unreasonable to offer it to patients with very severe headache after 24 – 48 hours.
Conclusion
Spinal puncture is an important cause of iatrogenic morbidity in the form of post-dural puncture headache. The incidence of these headaches can be reduced by using thin atraumatic needles in the procedure, however.
