Chest pain is a common cause of hospitalisation. Sometimes a conspicuous examination finding can lead to the wrong conclusions and cause important findings of major clinical importance to be overlooked.
A man in his early 20s was admitted to the medical department of the local hospital because of almost two days of chest pain. The pain started in the centre of his chest and gradually moved over to the left. It was exacerbated by deep inhalation and lying on his left side. The patient had no other symptoms. Two weeks prior to admission he had had similar chest pain that had ceased spontaneously, and a month before admission he had had slight cold symptoms. Apart from this the patient was well and used no medicines or intoxicants. There was no history of cardiac disease in the family.
Chest pain often results in immediate hospitalisation. In some patients it is a symptom of a serious pathological change, while in others it may be due to benign, self-limiting conditions. The causes can be divided into cardiac and non-cardiac. As a rule, young age points to a non-cardiac cause of the pain rather than, for example, coronary heart disease, while pneumothorax and viral pericarditis are frequent causes in the age group 20 – 40 (1).
The patient’s general condition was good, but he was in slight pain. When examined the patient presented as nonfebrile with normal vital parameters including equal pulse in upper extremities and both groins. Weak respiratory sounds were heard, equal over both sides, and no adventitious sounds. On auscultation of the heart, a pronounced friction rub sound was heard over the whole precordium. The sound almost drowned out the heart sounds. A gurgling sound from the apex was also heard in left lateral decubitus. This gurgling sound was audible even without a stethoscope. Ictus was palpated in the mid-clavicular line. The rest of the clinical examination revealed normal conditions. Electrocardiogram (ECG) showed normal sinus rhythm with no signs of ischaemia or other pathological conditions.
Because of the friction rub sound, we considered pericarditis the most probable diagnosis, whereas the chest pains that were exacerbated by deep inspiration, combined with the patient’s age and general condition, also made pneumothorax a differential diagnosis. The friction rub could also be due to pneumomediastinum or pleuritis. Pulmonary embolism can cause respiration-dependent pain, and both pulmonary embolism and dissecting aortic aneurism can in rare cases cause adventitious sounds reminiscent of the sound that is heard with pericarditis (2, 3). Thoracic pain in connection with deep respiration may also be due to myalgia, but we regarded this as a less probable diagnosis because of the adventitious sounds. One of the first things we wanted to determine was whether the heart was affected, and ECG and telemetry, combined with blood tests, were natural choices. A chest X-ray was also regarded as a natural part of the assessment.
We ordered blood tests in the form of infection tests (C-reactive protein, sedimentation rate and leukocytes), electrolytes, haemoglobin, D-dimer (fibrin degradation product) and myocardial enzymes (troponin I and CK-MB). Chest X-rays were taken during inspiration and expiration to check for pneumothorax. Samples were also taken for serological testing, having in mind myocarditis of viral genesis (cytomegalovirus, enterovirus, Epstein-Barr virus, adenovirus or parvovirus B-19).
The patient was observed overnight with telemetry, with no signs of rhythm disruptions or ischaemia. Echocardiography was performed the next day and showed no definite signs of pericarditis. Chest X-rays were described as normal. All the blood test results were within the reference ranges. Serology showed signs of previous infection with cytomegalovirus.
He was diagnosed as having pericarditis of assumed viral origin and was treated with anti-inflammatory drugs in the form of naproxen. The day after admission he was discharged in good general condition and virtually free of pain.
Because of normal vital parameters and no dyspnoea, more serious diagnoses such as aortic dissection and pulmonary embolism were regarded as improbable. Equal pulses in both arms and groins also weighed against aortic dissection. D-dimer, which almost always rises with pulmonary embolism and as a rule with aortic dissection, was also normal in our patient. The normal X-rays of the lungs made pneumothorax or pneumomediastinum unlikely diagnoses. Although echocardiography revealed no definite signs of pericarditis, the adventitious sound that could be clearly heard over the whole precordium were decisive for the diagnosis pericarditis.
A short time after discharge the patient took a long flight. Shortly after arrival the patient again developed chest pain and was admitted to hospital. Pneumothorax was found.
This information led to a renewed review of his stay in our hospital. Close inspection of the X-rays of the lungs showed a small, apical air cap on the left side which measured about 12 mm during expiration (Fig. 1).
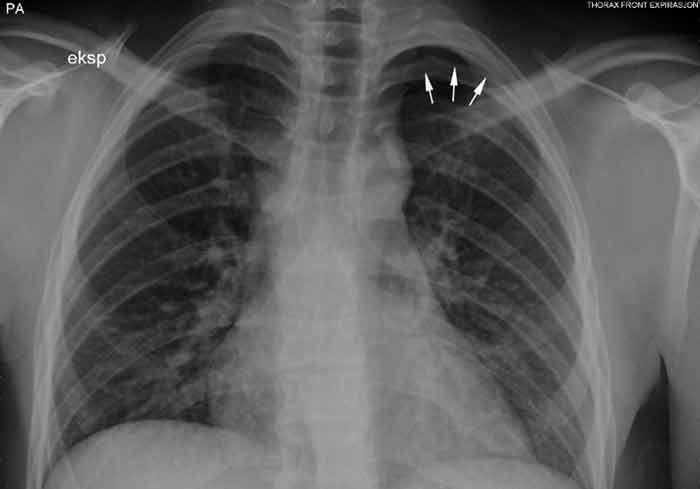
Figure 1 Chest X-ray taken during expiration shows a 12 mm apical air cap on the left side.
A PubMed search with the key words pericarditis and pneumothorax identified articles that described the condition «noisy» or «clicking» pneumothorax, a condition where patients have pneumothorax with the addition of pulse-synchronous adventitious sounds from the chest (4 – 8).
Our patient’s symptoms are consistent with the symptoms described in the literature: chest pain, exacerbation on inspiration, «gurgling» sounds from the apex area and dispersion of this sound over the precordium. We therefore assume that the patient had such «noisy» pneumothorax and not pericarditis, as first assumed.
Discussion
There are several similar cases described in the literature where the patient was first given the diagnosis pericarditis and subsequently pneumothorax after a review of the X-ray pictures (4 – 8). Pneumothorax is an accumulation of air in the space between visceral and parietal pleura. Pneumothorax is called spontaneous if no triggering events can be identified. Studies from both Norway and the USA have shown that the condition is far more prevalent in men than in women (9, 10). Spontaneous pneumothorax normally arises as a result of an apical, subpleural emphysema bladder bursting. Smokers are at greater risk, and persons with spontaneous pneumothorax have a tendency to be taller than control patients (11, 12).
Noisy pneumothorax has been described several times in the literature in the form of case histories (4 – 8). One material reports that less than 1 % of the spontaneous pneumothorax cases are noisy pneumothorax, another up to one of six cases (5, 6).
The sound that is heard with noisy pneumothorax is described in the literature as scraping, bubbling, clicking or crunching. This sound is also called Hamman’s sign, and was initially described by Louis Hamman in 1937 in connection with pneumomediastinum (13). Pneumomediastinum is air in the mediastinum and can develop spontaneously, following an injury or in connection with a change in intrathoracic pressure as in an asthma attack, vomiting or a visit to a dentist where compressed air is used (14). In cases of adventitious sounds over the precordium, it is particularly important to consider the possibility of air in the mediastinum. In some cases of pneumomediastinum, subcutaneous emphysema is also found.
The friction rub sound with noisy pneumothorax occurs when the heart’s mechanical work causes movement of small quantities of air intrapleurally (7). The sound occurs most frequently with left-sided, small apical air caps and is most clearly audible in left lateral decubitus position. Only one case of right-side pneumothorax with such adventitious sounds has been described (6). Phonographic studies have shown that there are often several adventitious sounds, both systolic and diastolic, and that they can vary with the respiratory cycle and body position (15).
Normal vertical chest X-rays with posterior-anterior radiation is normally enough to make the diagnosis pneumothorax. X-rays taken during expiration are not recommended as part of an ordinary routine assessment (12). Small air caps can be difficult to detect, and it is easy to overlook a small pneumothorax, particularly if one is not actively looking for it. On vertical images, an apical, hyperclear zone is usually seen with a visible contour of visceral pleura and absent vascular markings peripherally. On decubitus images it may be more difficult to diagnose a pneumothorax since air rises up to the highest point and then collects anteriomedially against the base of the lung (16).
A pneumothorax can be treated in different ways, depending on the cause, symptoms, size of the air cap and whether it is a recurring condition. There are no special guidelines for treating noisy pneumothorax. The British Thoracic Society has drawn up general guidelines for treating spontaneous pneumothorax (12). According to these guidelines, patients with small air caps can be monitored as outpatients, while recurring air caps should be treated with aspiration, oxygen, drains or pleurodesis, depending on the size, number of recurrences and symptoms.
Having had spontaneous pneumothorax can have consequences for choice of occupation and leisure activities. These patients should be protected from barotrauma (rapid changes in air pressure). Previous spontaneous pneumothorax is a contraindication for diving with compressed air tanks if it has not been treated with bilateral pleurectomy (17). The danger lies in the fact that air expands when the pressure drops as the diver rises to the surface. Patients who have had pneumothorax should also be careful about air travel (18). The air pressure in the cabin is lower than the pressure on the ground, and this can cause a pneumothorax to expand. The expansion of air at lower pressures can also make the patient disposed to develop tension pneumothorax, which is a medical emergency situation. A tension pneumothorax develops when a one-way valve forms that allows air into the pleural cavity but not out again. The British guidelines recommend chest X-rays to confirm full reversal of pneumothorax before air travel, and waiting about a further seven days after normalisation (18).
In our patient, the air travel itself may have contributed to both the recurrence of the pain and to the diagnosis. The low barometric pressure in the cabin may have caused the air cap to expand during the flight.
It is easy to be wise after the event. Noting that the friction rub was synchronous with respiration or pulse might have helped to some extent with the diagnosis. Whereas friction rub caused by pericarditis or noisy pneumothorax will be synchronous with the heart rhythm, the adventitous sound associated with pleuritis, for example, stops if the patient holds his or her breath. It is of course regrettable that the air cap apically on the chest X-ray during expiration was not detected on the first review of the X-rays. On the other hand, this has been a reminder to us of how important it is to keep updated with respect to more uncommon diagnoses. In our case the patient’s condition could have taken a serious turn in connection with the flight, many hours long, that he took after discharge from our department. A correct diagnosis initially would have prevented this.
