Advice from general practice
In the pilot study, 51 GPs responded (33 %), while 302 GPs (26 %) responded to the main study that was sent to doctors ordering analyses from six major Norwegian laboratories (the response rate varied from 16 % to 39 %). The results of the pilot study and the main study were largely consistent. Ninety per cent of the doctors reported that at least one analysis required notification. Electrolytes and some haematological parameters were most commonly indicated (see Fig. 1). The limits for immediate notification proposed by the doctors are shown in Table 2. Many doctors were uncertain as to whether the various test results required notification, and which limits should be used, which they indicated both by ticking the square «Don’t know» and by stating this in the comments field on the form. Even when doctors wanted to be alerted about a particular analyte (Fig. 1), they often did not want to suggest limits at which an alert should be issued (Table 3). This was particularly noticeable for one analyte (D-dimer); 45 % of doctors wanted to be notified, but far fewer (18 %) wanted to propose a critical limit themselves.
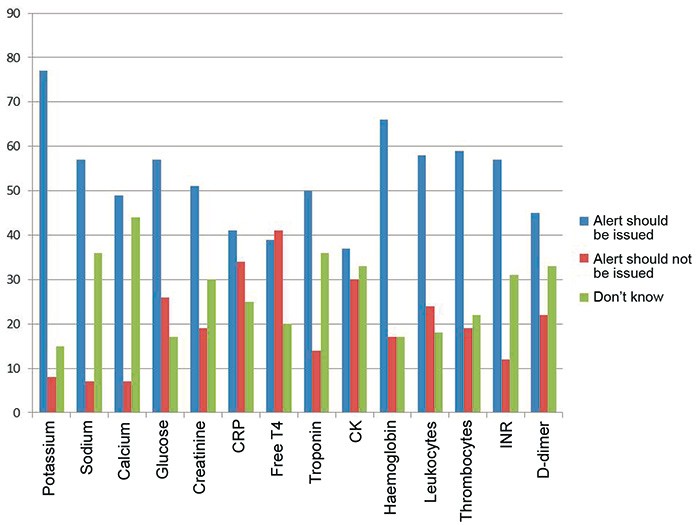
Figure 1 The percentage of GPs (n = 302) who believe that notification should or should not be given of pathological results for various biochemical analyses
In the main survey, the doctors were asked about their view of this type of service. Sixty-two per cent of the doctors had been contacted by a laboratory because of an abnormal test result, and they rated the benefit of this, on a scale from 0 (of no use at all) to 10 (very useful), as having an average value of 8.9. Fifty-four per cent had experienced that the laboratory contacted Accident and Emergency A&E or other medical personnel regarding an abnormal test result when they themselves were unavailable, and this service was rated as having an average value of 8.3 (same scale as above). Most GPs felt that the primary doctory, or failing that, A&E, should be notified if the laboratory discovers an abnormal test result (see Fig. 2).
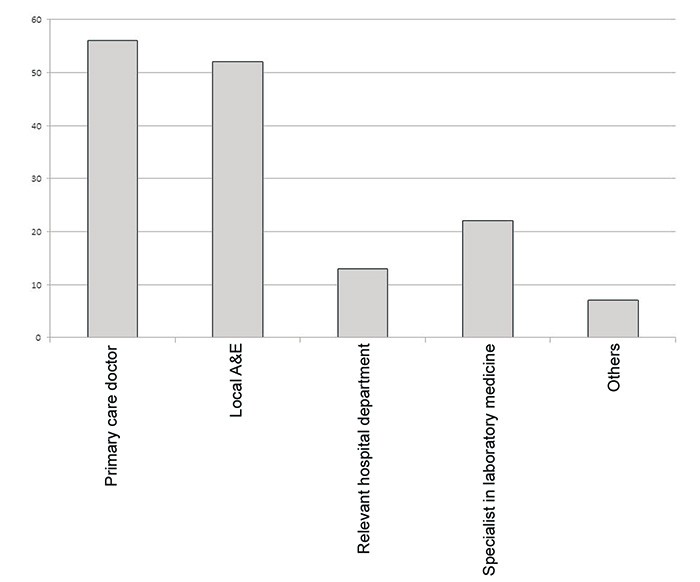
Figure 2 Percentage of GPs who proposed various authorities the laboratory should contact when they discover abnormal laboratory results. Some participants suggested several options.
Significance of the critical limits for patient outcomes
For many analytes (e.g. troponins, CRP, D-dimer) there is no definitive concentration that constitutes a significant acute health risk, and in such cases the clinical picture will be more of a deciding factor than the actual analytical result. In the case of other analyses (e.g. electrolytes) patients may have very abnormal or life-threatening values without there being any clear clinical symptoms. For such analyses there may be documentation for when a concentration is associated with a serious condition requiring treatment.
Sodium
With chronic hyponatraemia, symptoms do not usually occur at serum sodium concentrations of over 120 mmol/l (11 – 13), while if there is a rapid fall (less than 24 hours), nausea and faintness occur already at concentrations of less than 125 – 130 mmol/l. If the sodium concentration in the blood falls further (below 115 – 120 mol/l), life-threatening complications may arise (11, 14 – 17). Serious symptoms of hypernatraemia usually do not occur before the sodium concentration in the plasma/serum exceeds 158 mmol/l. Studies have shown that clinics rapidly react and take action if sodium concentrations of less than 120 or more than 155 mmol/l are found (18).
Potassium
In cases of severe hypokalaemia (defined as potassium < 2.5 mmol/l (19)) patients may experience muscular weakness and muscular cramps, in addition to ECG changes and cardiac arrhythmias (20). However, cardiac arrhythmias are rare in patients without known comorbid heart disease (19). Severe hypokalaemia may result in rhabdomyolysis and paralysis (21). Hyperkalaemia may result in muscular weakness, paralysis, severe cardiac arrhythmias and death. The risk of arrhythmia is higher for potassium values greater than 6.0 mmol/l, but is particularly high when potassium is > 6.5 mmol/l (22). There is evidence that patients with renal failure and chronic hyperkalaemia are less susceptible to fatal arrhythmias than patients who develop acute hyperkalaemia (23).
Phosphate
Phosphate deficiency can cause symptoms stemming from the central nervous system, musculature and heart (24). Serious symptoms do not usually develop before the serum phosphate concentration is less than 0.3 mmol/l (25, 26).
Calcium
There is no clear correlation between calcium concentration and symptoms. The degree of seriousness is determined primarily by how rapidly the hypo- or hypercalcaemia develops. (27). Tetany seldom occurs before total calcium falls below 1.9 mmol/l (28) and in rare cases low total calcium may result in serious arrhythmias (28). Chronic moderate hypercalcaemia with calcium values of 3.0 – 3.5 mmol/l may be tolerated relatively well, while the patient may become serious ill, possibly with clouded consciousness, if the hypercalcaemia develops rapidly. Patients with total calcium of over 3.5 mmol/l must be hospitalised immediately and treated, irrespective of what symptoms they might have (29). In rare cases, severe hypercalcaemia may increase the risk of both supraventricular and ventricular cardiac arrhythmias (30).
Magnesium
Severe hypomagnesaemia is defined as magnesium of less than 0.5 mmol/l (31) and is associated with ventricular arrhythmias, particularly in myocardial infarction patients (32). In contrast to hypomagnesaemia, hypermagnesaemia is rare (33). Typical symptoms are neuromuscular (34 – 38), cardiovascular (34, 36, 38) and metabolic (34, 39, 40). The first, mild symptoms of hypermagnesaemia can be observed with magnesium concentrations in the plasma/serum of over 2.0 mmol/l, while magnesium concentrations of over 3.0 mmol/l may extend the atrioventricular conduction time and concentrations of over 5 mmol/l may cause paralysis and cardiac and respiratory arrest (36, 41).
Glucose
Diabetic ketoacidosis and hyperosmolar, non-ketotic hyperglycaemia may by definition occur at glucose values of 13.9 and 33.3 mmol/l respectively (42). One study shows that the average serum glucose level of adults with diabetic ketoacidosis is 25.7 mmol/l (SD 0.9), while the corresponding value in children is 28.0 mmol/l (SD 1.4) (43).
Leukocytes
It is difficult to find documented risk of infection associated with a low leukocyte concentration in isolation. When about 1100 samples with a neutrophil granulocyte concentration of from 0.4 to 0.6·10⁹/l were tested, the leukocyte concentration varied from 0.5 to 5.5 · 10⁹/l. In 90 % of these samples, the leukocyte concentration was < 3.0·10⁹/l. Thus the leukocyte count can vary very considerably with neutropaenia, so the quantity of neutrophil granulocytes should be studied in this situation. The acute health risk represented by a high leukocyte count alone is associated with the risk of leukostasis, tumour lysis syndrome and disseminated intravascular coagulation (DIC) in connection with malignant blood diseases (44). These conditions are rare with leukocytes < 100· 10⁹/l.
Neutrophil granulocytes
The risk of infection with a low concentration of neutrophil granulocytes in the blood depends largely on what reserves the patient has in the bone marrow. There is little correlation between risk of infection and granulocyte concentration in patients with leukopaenia and otherwise normal bone marrow (45). In cancer patients with therapy-induced bone marrow depression, the risk of infection increases when the granulocyte count falls below 1.5·10⁹/l. At concentrations of < 0.5·10⁹/l the risk of infection increases substantially and isolation is called for (46).
Thrombocytes
There is no evidence of any acute risk of major bleeding in cases of thrombocytopaenia before the thrombocyte concentration is < 15 ·10⁹/l (47, 48). Thrombocytosis (> 500 ·10⁹/l) results in a somewhat higher risk of thromboembolic episodes (49). Values > 1000 ·10⁹/l imply a somewhat increased risk of acquired von Willebrand syndrome and of bleeding (50). Thus there is little acute health risk associated with thrombocytosis.
PT-INR
The risk of bleeding complications increases with rising PT-INR values. Studies have shown that the relative risk (number of incidents divided by the total number exposed compared for two cohorts) of major bleeding events increases by about 1.4 per unit increase in the PT-INR (51). For INR values of over 4.5, the relative risk of bleeding complications thus increases to about six (52). Another study shows that the odds ratio (number of patients with events/number of patients without bleeding events compared for two cohorts) for intracranial haemorrhage first increases when the PT-INR exceeds 4.0, and at a PT-INR of about 5, the odds ratio can be estimated at approximately five (53).