Congenital hearing loss is a common congenital condition which occurs in 1 – 3/1 000 newborns (1 – 3). In Norway, a prevalence of 1.0 – 1.6/1 000 has been detected, with fewer screened infants than in our study (4, 5). In comparison, the mandatory neonate screening for hypothyreosis and Følling’s disease (phenylketonuria) was in 2012 expanded to encompass 23 serious congenital diseases that have a total prevalence of 1/1 700 (6). In 2006, the Directorate of Health recommended that all newborns should be screened for otoacoustic emissions (OAE) before discharge from the maternity and neonate intensive care unit (7) (Box 1). As early as 1998 there was consensus among European audiologists on the introduction of hearing screening of neonates (8). The Directorate of Health recommends that otoacoustic emissions be repeated up to two times in the maternity ward and neonate intensive care unit before an examination with automated auditory brainstem response audiometry (AABR) is undertaken. This two-step model provides a sensitivity of 0.92 and a specificity of 0.98 (9).
BOX 1
Methods for hearing screening
-
Otoacoustic emissions (OAE)
-
Measurement
of the auditory response from the cochlea to a broadband click of 75 – 80 dB in the auditory canal. This stimulates the outer and inner hair cells and basilar membrane to produce a movement that spreads back through the middle ear and the eardrum similar to sound to a microphone. In screening, the response is processed automatically with the response alternatives «pass» (otoacoustic emissions are detected) or «refer» (no otoacoustic emissions are detected). The procedure can be undertaken by personnel with no audiology competence after some guidance. In the case of stationary otoacoustic emissions testing in the hearing centre, the response to frequency levels is measured by audiology personnel.
-
Auditory brainstem response audiometry (ABR, BRA)
-
Automated auditory brainstem response audiometry (AABR)
-
The
methodology is the same as in auditory brainstem response audiometry under narcosis, but with automated processing of the response. The response alternatives are the same as in otoacoustic emissions testing. The procedure can be undertaken by personnel with no audiology competence after some guidance and requires no sedation or narcosis.
Early diagnosis and initiation of treatment before the child is six months old is crucial. Children with congenital hearing loss may have problems in, for example, learning of grammar, word order, understanding of concepts and vocabulary (10, 11). Children with hearing loss detected by screening at the neonatal stage have better cognitive development, social adaptation, gross motor skills and quality of life than children whose hearing loss is detected by distraction testing when they are approximately nine months old (12). Low educational level, increased behavioural problems and difficulties in psychosocial adaptation are also associated with hearing loss in children (13).
Genetic causes of sensorineural hearing loss occur in up to 60 % of children with congenital hearing loss (14). A prevalence of 45 % was found in a Norwegian dataset (15). Approximately 80 % of the non-syndromic cases have an autosomal recessive aetiology, and mutations of the GJB2 gene on chromosome 13, which codes for the protein connexin 26, are responsible for half of these (16).
Known risk factors for hearing loss are presented in Box 2 (10). Risk factors are present in approximately 50 % of all children with hearing loss.
BOX 2
Risk factors for hearing loss in children (10)
Concern among guardians about hearing loss
Childhood hearing loss in the family history
Admission to neonatal intensive care unit > 5 days or mechanical ventilation, ototoxic medication, total transfusion or extracorporeal membrane oxygenation (ECMO)
Intrauterine TORCH infection, especially cytomegalovirus
Craniofacial malformations, especially if related to the ears
Syndromes associated with permanent hearing loss: Jervell and Lange-Nielsen’s syndrome, DiGeorge’s syndrome, Schwartz-Jampel’s syndrome and others
Syndromes associated with progressive hearing loss: neurofibromatosis, osteopetrosis, Usher’s syndrome
Neurodegenerative diseases, e.g. Hunter’s syndrome
Postnatal infections, especially bacterial meningitis
Head traumas with hospitalisation
Chemotherapy
Auditory neuropathy may occur in 5 – 19 % of newborns with hearing loss and may be progressive and/or late-onset (17 – 18). These children have normal function in the outer hair cells of the cochlea and normal otoacoustic emissions, but an abnormal neural response from the cochlea to the brainstem. Auditory neuropathy can be diagnosed by automated auditory brainstem response audiometry or auditory brainstem response audiometry under narcosis (ABR). Auditory neuropathy results in poorer perception of spoken language than what is indicated by the audiogram, and the children have particular problems in noisy environments (19). If parents are concerned about a child’s hearing, it is essential to be aware of this condition (10).
Since 1 January 2000, hearing screenings have been routinely undertaken at the maternity and neonate intensive care unit at Sykehuset Østfold. The objective of this study was to determine the prevalence of congenital hearing loss in a large-scale Norwegian dataset.
Material and method
Since 1 January 2000, all newborns in the maternity and neonatal intensive care unit at Sykehuset Østfold have been offered hearing screening with otoacoustic emissions, and children born in the period 1 January 2000 – 31 December 2009 were included. In the maternity and neonatal intensive care unit, data have been collected from paper-based documentation that covers the entire period. Up to and including April 2008 only children with no emissions bilaterally were referred to the hearing centre. From that time, children with unilateral lack of emissions have also been referred. In the hearing centre, data have been collected from paper records for the period 2000 – 01 and electronic records (DIPS) for the period 2002 – 09. Follow-up was undertaken until 31 December 2013.
The screening was undertaken upon discharge from the maternity ward in the child’s second day of life or upon discharge from the neonatal intensive care unit. The test outcome is automated, and the display shows either «pass» or «refer» (no emissions: refer for further examination). If a child was discharged from the maternity ward before screening or a test undertaken before discharge showed «refer», a new test was undertaken a few days later at the outpatient maternity clinic.
During the first two years, the number of parents who did not want their child to be examined was recorded. This was not undertaken during the last eight years of the period.
We also registered patients with a late-detected hearing loss. These were children who had normal results on the neonatal screening, but who later had been referred to the hearing centre with a hearing-loss issue from the mother-and-child clinic, general practitioner or an otorhinolaryngologist in private practice.
Screening equipment
In the maternity and neonatal intensive care units, screening was undertaken with Madsen EchoScreen and Madsen AccuScreen. These are portable, automatic instruments that measure transient otoacoustic emissions (TEOAE). A hearing loss in excess of 30 – 35 dBHL results in no emissions. We refer to previous descriptions of the technology and documentation practice (4).
At the hearing centre, stationary otoacoustic emission equipment was also used (ILO 88 and later ILOV6 Ez-Screen ver2, Otodynamics). If no emissions had been detected after one to three otoacoustic emission tests, the child was tested with the aid of automated auditory brainstem response audiometry (Madsen AccuScreen) during sleep. This procedure was brought into use from 1 November 2002, and measures electrical activity in the brainstem as a response to stimulation with a clicking sound. After a clinical assessment by an otorhinolaryngologist, the child was further referred to auditory brainstem response audiometry under narcosis (Otometrics Chartr EP200). As a general rule, this was undertaken in our hospital only after the age of six months after an assessment of the anaesthesiological risks involved, but would also be undertaken at an earlier age in special cases (see Discussion). During the period before automated auditory brainstem response audiometry was brought into use, children with no emissions after repeated testing were referred directly to auditory brainstem response audiometry under narcosis.
Ethics
This is a quality-assurance study that has been approved by the Norwegian Social Science Data Services (NSD) and undertaken with salary funding from the Quality and Research Department at Sykehuset Østfold. The registrations of children with hearing loss have been anonymised, and only information relevant for the purposes of the study has been retrieved through the systematic review of data in patient records and the hearing centre.
Statistics
We have used the chi-square test for estimation of differences in proportions and the t-test for differences in average values. P-values < 0.05 were regarded as significant.
Results
Screening for otoacoustic emissions
Measurable otoacoustic emissions were present in 93.5 % of the children at the first test. This proportion increased to 98.1 % after 2 – 3 tests in the maternity ward, the neonatal intensive care unit or outpatient maternity clinic. Among all neonates during the first two years, there were 69 (1.2 %) children whose parents did not want the examination to be undertaken. In the course of ten years, altogether 568 (1.9 %) children were referred to the hearing centre by the maternity ward, neonatal intensive care unit or outpatient maternity clinic because of absence of emissions (Figure 1).
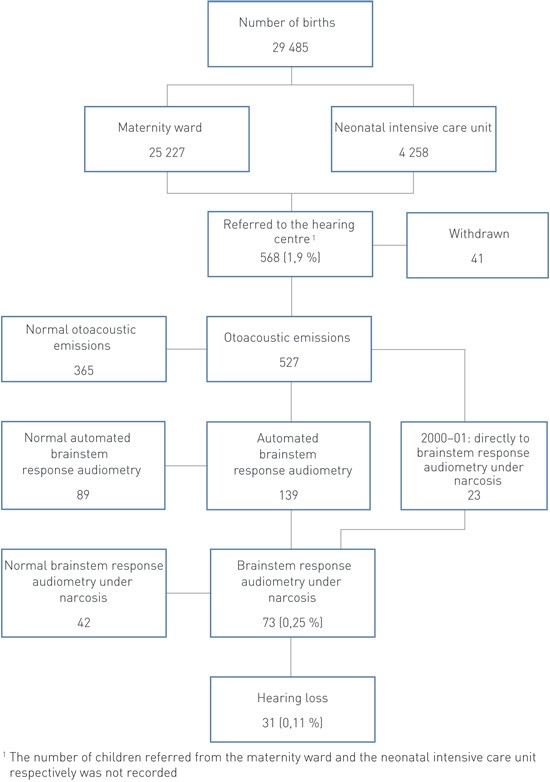
Figure 1 Hearing screening of newborns in Østfold county 2000 – 09 with the aid of otoacoustic emissions and further examination at the hearing centre
Absent emissions at the neonatal screening
Of the 568 children who were referred to the hearing centre, there were 19 children (3.4 %) who did not turn up, and another 22 children (3.9 %) later discontinued the examination. None of these have later been referred to the hearing centre. After 1 – 3 tests with stationary otoacoustic emission equipment, altogether 365 (64 %) children had normal emissions. During 2000 – 01, a total of 23 of the remaining 162 were referred directly for auditory brainstem response audiometry under narcosis, and 139 have since 2002 been examined with automated auditory brainstem response audiometry. Of these, 89 had normal hearing, while 50 were referred for auditory brainstem response audiometry under narcosis. Among this total of 73 (12.3 %) children, altogether 31 (5.5 %) suffered from hearing loss while 42 had normal hearing. Four of them had a unilateral hearing loss (Table 1). After the introduction of automated auditory brainstem response audiometry, the proportion of children examined with the aid of auditory brainstem response audiometry under narcosis was significantly reduced (23/5 633 (0.41 %) in 2000 – 01 and 50/23 852 (0.21 %) in 2002 – 09) (p < 0.001).
|
Table 1 Hearing loss in children in Østfold county 2000 – 09 after absent and normal emissions in the neonatal screening. Number, unless otherwise specified
|
|
Absent emissions, n = 31 (0.11 % of those screened)
|
Normal emissions, n = 10 (0.03 % of those screened)
|
|
Girls
|
16
|
2
|
|
Unilateral hearing loss
|
4
|
0
|
|
2000 – 04
|
|
|
|
Number of children
|
9
|
8
|
|
Age in months at diagnosis, median (range)
|
13 (7 – 74)
|
35.5 (24 – 86)
|
|
2005 – 09
|
|
|
|
Number of children
|
22
|
2
|
|
Age in months at diagnosis, median (range)
|
6 (3 – 23)
|
36 (18 – 54)
|
|
|
|
|
Treated with hearing aid
|
23
|
6
|
|
Treated with a cochlear implant
|
7
|
4
|
|
First-degree relative with hearing loss
|
9¹
|
4
|
|
Admitted to neonatal intensive care unit
|
5
|
4
|
|
Presence of other risk factors²
|
5
|
5
|
|
[i]
|
Six of the children who had a congenital hearing loss (19 %) received this diagnosis before they were five months old, and eleven children (35 %) before they were six months old. The median age of diagnosis was 8.5 months (range 3 – 74) for the entire ten-year period. In 2000 – 04, altogether nine children were discovered to have hearing loss at the median age of 13 months, and in 2005 – 09, a total of 22 children were diagnosed at the median age of six months. The annual variation in number amounted to 0 – 6 children. Their average age was 22.6 (SD 19.2) and 11.2 (SD 6.3) months respectively (p < 0.01). Treatment was initiated within one month after the examination in all of them. Six of the seven children who received a cochlear implant were first supplied with a hearing aid.
The positive, predictive value for referral to the hearing centre for the entire period was 0.055. Correspondingly, the positive, predictive value for referral for auditory brainstem response audiometry under narcosis was 0.42. Using otoacoustic emissions as a screening method produced a specificity of 98.1 %, while the proportion of false positives amounted to 1.8 %.
Hearing loss in children with normal results in the neonatal screening
Ten (0.03 %) children who were born during the period were diagnosed with bilateral hearing loss after having shown normal results in the neonatal screening. The median age of diagnosis for this group was 36 months (range 18 – 86) for the entire period. The youngest of these children was born in 2008.
Hearing loss in children in the neonatal intensive care unit
Five of the children who had hearing loss at the neonatal screening and four of the late-detected cases had been admitted to the neonatal intensive care unit. These nine children accounted for 0.21 % of all admissions to the neonatal intensive care unit. The other 32 children had been admitted to the maternity ward and accounted for 0.13 % of all admissions there (no significant difference). The median age of diagnosis for children admitted to neonatal intensive care was 23 months, compared to ten months for the others. The number of children who were referred to the hearing centre from the neonatal intensive care unit was not recorded, and the relative risk can thus not be estimated for this group.
Heredity
Of the 31 children who had a hearing loss that was detected by screening, altogether nine (29 %) children had first-degree relatives with varying degrees of hearing loss. Of these nine children, four (44 %) tested positive for a mutation of the GJB2 gene, which codes for the protein connexin 26 (CX26). One child without first-degree relatives with hearing loss also tested positive for mutation of the GJB2 gene. The review of patient records yielded no information regarding genetic testing of the remaining children.
Among those children who had normal emissions at the neonatal stage but were later diagnosed with hearing loss, four of ten (40 %) had first-degree relatives with hearing loss, but none had mutations of the GJB2 gene.
Discussion
Our study of congenital hearing loss in a large Norwegian population of neonates shows a prevalence corresponding to international figures. The proportion of late-detected cases within our period of follow-up is also as expected in light of other studies. There was a decrease in the median age of diagnosis during the period, and the introduction of automated auditory brainstem response audiometry reduced the need for brainstem response audiometry under narcosis. The degree of coverage of the screening amounted to 98.8 % during the first two years, well inside the recommended degree of coverage of 98 % (10). In the ensuing years the degree of coverage was not recorded, but we have no reason to assume that it has declined.
The national and international recommendations stating that hearing loss should be diagnosed before the age of three months and treatment should be initiated before the age of six months were not reached, although we achieved a considerable reduction of the median age of diagnosis, from 13 to six months from the first to the second half of the period. In most cases, the delay in the diagnosis was due to parents who did not turn up for further examination in spite of repeated calls. This was especially the case during the first period, but occurred also during the second. The hearing centre has started to place a clearer emphasis on the likelihood of hearing loss when emissions are absent, as well as on the consequences of incomplete diagnosis and treatment.
Although eleven (35 %) children were diagnosed in the course of their first six months, there were 20 who were diagnosed too late for initiation of optimal treatment and habilitation programmes. Ten of these children were older than 12 months. The six children who were diagnosed before six months of age were examined by auditory brainstem response audiometry under narcosis after considerable pressure from parents who were either hearing-impaired or deaf themselves, or when there were strong indications of deafness after automated auditory brainstem response audiometry had been undertaken. In light of practices in which auditory brainstem response audiometry is undertaken under narcosis only from the age of six months onwards for anaesthesiological reasons, initiation of treatment within the recommended age cannot be achieved. In recent years we have therefore made use of auditory brainstem response audiometry during natural sleep, in practice also before the age of three months. This has enabled earlier diagnosis and initiation of treatment before the age of six months. Other studies have shown a median age for initiation of treatment of 2.5 months and eight months respectively (20, 21). Both these studies, as well as our own material, show that the proportion of children with normal emissions increases in pace with the number of otoacoustic emissions tests. One study found that 0.8 % of those screened were referred for auditory brainstem response audiometry after having undergone up to four otoacoustic emissions tests (20). In our study, 0.25 % of the children were referred to auditory brainstem response audiometry under narcosis after having gone through up to five otoacoustic emissions tests and automated auditory brainstem response audiometry.
The proportion of children who developed hearing loss after a normal neonatal screening is comparable to findings in a study that had a follow-up period of nine years and in which the median age of diagnosis was 26 months (22). The follow-up period is shorter in our study, where the youngest children only had 48 months of follow-up, and the eldest child was 86 months when diagnosed. Relocations out of the county also introduce a degree of uncertainty regarding the number of late-detected hearing loss cases.
Seven of the ten late-detected children had at least one risk factor for hearing loss. All of the nine children who had been admitted to the neonatal intensive care unit received treatment with aminoglycosides. This is the standard empirical treatment in cases of neonatal sepsis in Norwegian as well as most Western neonatal intensive care units. Because of the risk of hearing damage, the serum level is monitored closely in these children. Risk factors are assumed to be present in 10 – 30 % of all newborns, while 50 % of all children diagnosed with hearing loss have no known risk factors (Box 1) (10). Our figures correspond well with this: a total of 13 (42 %) of the 31 children who had no emissions in the neonatal screening had risk factors. Of these 13, nine had first-degree relatives with hearing loss and five tested positive for connexin 26. Heredity may be involved in 60 % of cases, and connexin 26 accounts for 50 – 80 % of the non-syndromic recessive cases (10). The presence of hearing loss in close family, especially among late-detected children, most likely implies that closer follow-up of these will result in earlier diagnosis and initiation of treatment.
Until April 2008, children with unilateral hearing loss detected by otoacoustic emissions testing were not referred to the hearing centre, because it was believed that hearing in one ear was sufficient for language development. Unilateral hearing loss is a risk factor for bilateral hearing loss, and 10.6 % will have progression to hearing loss also in the other ear (23). Currently, children with a unilateral outcome are therefore followed up with annual otoacoustic emissions testing and automated auditory brainstem response audiometry until their hearing threshold can be measured by pure-tone or speech recognition audiometry. One child in our material suffered from unilateral hearing loss as a neonate and was diagnosed with bilateral hearing loss at the age of 20 months.
The Joint Committee on Infant Hearing recommends that children in maternity wards be screened either with the aid of otoacoustic emissions or automated auditory brainstem response audiometry in a one-step or two-step model. The most common practice is to use a two-step model with otoacoustic emissions, followed by automated auditory brainstem response audiometry as required. It is recommended that children who have been admitted to neonatal intensive care be screened with the aid of automated auditory brainstem response audiometry to enable diagnosis of auditory neuropathy, since otoacoustic emissions testing will not reveal this condition (24). If automated auditory brainstem response audiometry had been used in the neonatal intensive care unit it is conceivable that the four children with late-detected hearing loss could have been diagnosed at an earlier stage. The time required is on average somewhat longer for automated auditory brainstem response audiometry than for otoacoustic emissions testing, but children and their parents can be spared the burden of repeated checks and worries (25). A false-positive screening results in increased worry on the part of 3.5 – 14 % of parents, and 8 % of mothers report that they treat their children differently (e.g. talking louder, clapping their hands) (2).
Before the introduction of hearing screening for otoacoustic emissions, the median age of diagnosis of serious hearing loss in children was 2.5 years (7). It is satisfying to see that a considerable reduction in the age of diagnosis has been achieved during the first ten years of hearing screening in Østfold county, although we have failed to reach the goal set by the Directorate of Health of initiating treatment before the age of six months in the majority of cases. Screening for otoacoustic emissions is an effective method for children in maternity wards, while children in neonatal intensive care units should be screened with the aid of automated auditory brainstem response audiometry.
