Research projects that involve humans may produce intended (within the primary purpose of the study) as well as unintended results (outside the primary purpose of the study). The clinical implications of both types of results may vary from insignificant to potentially life-saving for the project participant. Several authors have discussed how such findings should be managed, including the views of the project participants (1) and those of the researchers (2), as well as various ethical and legal aspects (3 – 6). Although this issue is relevant to all areas of clinical research, the debate has mainly focused on genetic (3, 4, 7 – 9) and imaging studies (5, 10). There is no consensus regarding how the issue ought to be handled, although proposals suggest that information on such findings can/should be offered to the project participants, provided that the finding is reliable, that it is related to a serious affliction and that a treatment option is available (3, 4, 11).
In 2005, the Council of Europe presented an additional protocol concerning biomedical research to be added to the Convention on Human Rights and Biomedicine. The protocol provides elaborate regulations on biomedical research and refers explicitly to the duty of care (12): «If research gives rise to information of relevance to the current or future health or quality of life of research participants, this information must be offered to them. That shall be done within a framework of health care or counselling». Norway has signed the protocol, and it was used as background material for the preparation of the Health Research Act (13). Although Norway has not yet ratified the protocol and the duty of care was not explicitly referred to in the preparatory work for the Health Research Act, we may reasonably assume that this principle should be normative for Norwegian practice.
Good administrative practice implies a general requirement for documentation and traceability, and thus written documentation. Experience from the establishment of an internal control system for provision of diagnostic material for research purposes showed that the project documents often lacked information on how new, clinically relevant findings were to be managed (14, 15). This study was undertaken to investigate how the management of findings (intended as well as unintended) with clinical implications are generally documented in research projects that have received prior approval by a regional committee for medical and health research ethics.
Material and method
All research projects that had been approved by the ethics committee and were administratively associated with Oslo University Hospital with a starting date in 2011 were identified in the public database of the regional ethics committee (16) in June 2012. Normally, the application will include the application form, the project protocol and participant information/consent forms. Copies of these documents, along with the letter of approval by the committee, were therefore retrieved from the hospital’s information system for research administration. In cases where one or more documents were missing, an email was sent to the person in charge of the project with a request to provide the documents.
All projects were subsequently given an initial review, and only those that involved living humans and had actually been initiated were included in the study. The projects were categorised as patient-oriented or non-patient-oriented studies. This is s simplified version of a classification system used by the National Institutes of Health in the USA (17). In this context, patient-oriented studies refer to projects in which the researchers interact directly with people (irrespective of whether they are patients or not). The patient-oriented projects were further subdivided into four sub-types (Figure 1) (adapted from (17)). Finally, all project documents were reviewed with regard to the management of findings with possible clinical implications.
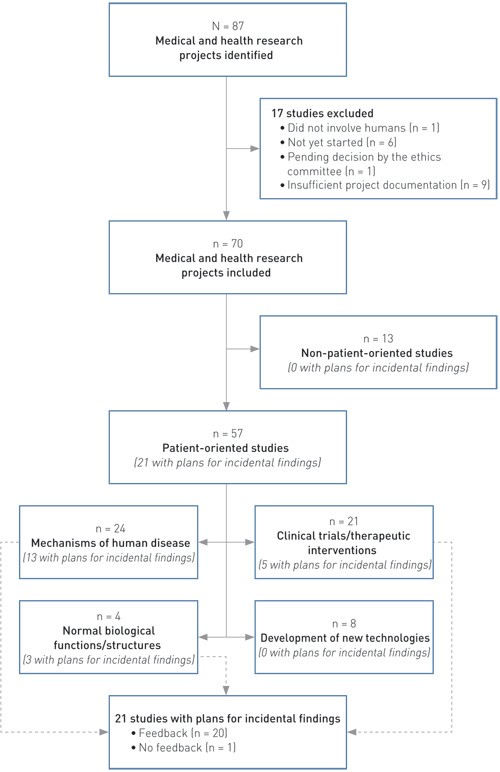
Figure 1 Characteristics of medical and health research projects that had been granted prior approval by the regional ethics committee, were associated with Oslo University Hospital and had a start-up date in 2011, reviewed with regard to their plans for managing findings with possible clinical implications
Results
Altogether 87 research projects with prior approval granted by the ethics committee, associated with Oslo University Hospital and with a start-up date in 2011 were found in the database. Of these, 17 projects were excluded because they did not involve living humans (n = 1), had not started even though they had been granted prior approval by the regional ethics committee (n = 6), had been amended and thus were still under assessment (n = 1), or because a complete set of project-related documents was unavailable (n = 9).
Of the remaining 70 projects, 57 were classified as patient-oriented and 13 as non-patient-oriented (Figure 1). Five projects (whereof one patient-oriented) had been granted exemption from the requirement for explicit consent, and no participant information/consent forms were therefore available for these. Of the 57 patient-oriented studies, 51 included inpatients (seven also included persons assumed to be healthy), four included non-hospitalised persons with known illness and two included persons assumed to be healthy. Use of biological material had been planned in 45 of the 57 patient-oriented studies (whereof 15 with genetic tests), and imaging techniques were to be undertaken in 21 cases.
In 21 of the 70 projects (30 %), information on the management of research findings with possible clinical implications was found in one or more project documents. In the remaining 49 projects, no such information could be found. All of the 21 projects that referred to this issue were patient-oriented studies (Figure 1). In all cases, the applicant him-/herself had referred to this topic in the initial application to the regional ethics committee. No projects were found in which the committee in its response to the applicant pointed out that such information was missing, requiring an elucidation of how research findings that had clinical implications were to be handled. In one case, however, in which the applicant had stated that no feedback of results was planned, the committee had added a comment saying that such feedback would be reasonable, provided that the finding had a bearing on the issue in question. With regard to the descriptions in the project documents pertaining to the management of findings with clinical implications, Table 1 shows the distribution between patient-oriented projects that involved diagnostic imaging examination or genetic testing and other patient-oriented projects.
|
Table 1 Number of patient-oriented projects that included a description of the management of findings with possible clinical implications in one or more of the project documents, sorted by whether the study involved imaging, genetic testing or other methods. Projects that had been granted prior approval by the Regional Committee for Medical and Health Research Ethics, associated with Oslo University Hospital and with a start-up date in 2011
|
|
|
Description in the project documents, n (%)
|
|
Projects with diagnostic imaging (n = 21)
|
9 (43)
|
|
Projects with genetic testing (n = 15)
|
6 (40)
|
|
Other projects (n = 21)
|
6 (29)
|
|
All patient-oriented studies (N = 57)
|
21 (37)
|
Among the 21 projects that provided written information regarding the management of findings with possible clinical implications, there was one project that included a clear statement to the effect that the project findings were deemed to be of no clinical significance, and no feedback would therefore be given to the participants. The other 20 projects stated that such feedback would be provided. Of these 20 projects, two stated that feedback was to be sent to the participants’ primary doctor. The remaining 18 projects did not explicitly specify how the feedback was to be provided. Based on the nature of these projects, it is reasonable to assume that the doctor(s) in charge of the study would provide the feedback.
For 13 of the 21 projects, the participant information/consent forms contained information regarding feedback of findings with possible clinical implications. The amount of information was mostly restricted to a few lines. Formulations such as «This study/test may produce findings that require clinical follow-up. If such findings are made, you will be informed» were common.
Discussion
The majority of the research projects that had been granted prior approval by the ethics committee and were investigated (49 of 70) did not include any information on the management of findings with possible clinical implications in its project documents. This is consistent with a similar international study, in which such information was absent in 45 of 85 research biobanks examined (18). This absence of plans for managing research findings with possible clinical implications in the project documents may have several causes:
The issue has not been considered.
The issue has been considered, but the likelihood of making such findings has been deemed insignificant and no plan has therefore been prepared.
The issue has been considered and a plan has been prepared, but not appended to the project documents submitted to the regional ethics committee.
The absence of written documentation does not necessarily mean that the researchers fail to follow up such findings adequately when they deem them to be of clinical relevance. Nearly all patient-oriented projects, which have the greatest likelihood of clinically relevant findings, were undertaken by doctors who recruited the participants from the ongoing clinical activities at the hospital. The project participants may thus have been offered completely appropriate follow-up, irrespective of whether this was stated in the project-related documents or not.
This investigation includes only one university hospital, and it cannot be assumed that the results are representative for other organisations that undertake medical and health research. However, the regional committees for medical and health research are public bodies, and good administrative practices call for equal treatment of all project applications. Since the committee apparently had no particular focus on the handling of findings with possible clinical implications with regard to the applications included in this study, we have reason to assume that the committee has followed the same practice in its review of project applications from other research organisations.
Risk can be regarded as a combination of the probability that a given incident will happen and the effect of this incident. Appropriate risk management requires a documentable process that includes identification, assessment and prioritisation of risks, as well as measures to reduce these risks as required (19). The likelihood of findings and the clinical implications of such findings will both depend on the type of research project in question. All of the 70 research projects included in our study involved examinations of humans, human biological material and/or health information. The majority of the projects involved direct interaction with project participants (mainly patients), as well as examinations of human biological material and/or imaging techniques. We may therefore assume that there was a real possibility of making findings that could have clinical implications for the participants. One could argue that those 21 projects that included written plans for how such findings were to be managed represented projects in which such findings were both highly likely and of substantial clinical relevance, whereas this was not the case for the remaining projects. However, many of the studies that involved imaging and/or genetic examinations made no reference to the issue in their documentation (Table 1). Varying practices among different researchers appear to be a more likely explanation of the observed difference in the frequency of references to this issue.
In a review article on ethical issues concerning provision of feedback or no feedback of genetic findings to project participants, Steinsbakk and Solberg concluded that «irrespective of the conclusion arrived at, the conclusion and the justifications must be communicated clearly and understandably to the participants. This is the unquestionable obligation of the researchers and the research institutions to the participants» (20). I share this view. In Norway, however, the Personal Data Act stipulates a general entitlement for individuals to receive information on how their personal data are handled, including the type of data that have been collected (21). Even if no feedback of research findings to individual project participants has been planned, the participants may request access to the data. Consequently, it will be expedient to make provisions for project participants to access all findings, and to plan the projects on this basis.
The researcher’s role is different from the clinician’s. Nor can we presume that all those who use information generated by medical and health research possess clinical knowledge and experience. Planning of how findings with possible clinical implications shall be managed does not mean that the individual researcher must assume a personal responsibility for following up such findings. The responsibility lies in considering what may come to light, who will assess the findings and how they should be followed up, if relevant. If this research is undertaken in a hospital environment, one solution could be that findings with possible clinical implications should be reported to the head of the department in which the biological material originated or in which the project participants were recruited (15).
In 2012, the Council of Europe’s steering committee for bioethics issued a manual for members of ethics committees. The manual states that project participants are entitled to receive health-related information that emerges as a result of research. It states further that the project application to an ethics committee must contain sufficient information to permit a thorough assessment (22). In December 2013, the Presidential Commission for the Study of Bioethical Issues in the USA issued a report on the handling of intended and incidental findings made in the context of clinical activity, research and direct-to-consumer testing. One of the commission’s five general recommendations was that those affected should be informed about the likelihood of such findings associated with specific tests or procedures. Moreover, they should be informed about any plans for information and management of the findings, including of the types of findings for which no such feedback is planned. With regard to research projects, the commission recommended that researchers prepare plans for the management of such findings and that these plans should be reviewed and approved by an ethics committee (23).
As written information on the plans for managing findings with possible clinical implications was absent from 49 out of 70 research projects, this will necessarily have reduced the opportunity of the regional ethics committees to assess them. Checklists are used for purposes of quality improvement in a number of areas, including medicine and peer reviews (24 – 26). Nevertheless, we cannot assume that professionals use such tools in practice (27). A clearly defined topic and an opportunity for autonomous professional assessment are crucial for users to accept a checklist (26). With regard to research findings with possible clinical implications, a practical approach could be to introduce a separate item for this issue on the form to be submitted to the regional ethics committee when the researcher applies for prior approval of the project. On the basis of his or her own professional assessment, the researcher may then describe how this issue will be managed and design the information in the participant information/consent forms accordingly. The committee will then be better equipped to assess the adequacy of the researcher’s proposal.
