In the workup of fever of unknown origin it is important to establish the patient’s travel history. Where have you been, and what did you do? This patient presents with fever, deteriorated general condition and non-specific airway and dermal symptoms. Recent travel in a tropical area and eosinophilia swiftly directed suspicions to a parasitic disease, but inadequate information about exposure made the initial workup more extensive than necessary.
Malaria should always be considered in febrile patients who return home from endemic areas. Plasmodium falciparum has an incubation period of up to four weeks (1), and in the present case the onset of the first fever episodes was within this time interval. The patient had used atovaquone/proguanil as malaria prophylaxis, but admits having forgotten some doses. He had slept indoors and used a mosquito net. Normal values for haemoglobin, platelets and reticulocytes and negative results for a rapid diagnostic test for malaria and thick smear test six weeks after the last possible exposure suggested that malaria was an unlikely diagnosis.
Typhoid fever is another serious imported disease that should be excluded at an early stage for travellers. The onset of this condition will normally be in the course of the first 1 – 3 weeks after the traveller’s return home (2). The patient reported dry cough, which is seen with typhoid and paratyphoid fever. Although the absence of fever, thrombocytopenia and leukopenia, and a weak acute phase reaction weigh against this differential diagnosis, blood cultures were obtained. They remained sterile.
Acute HIV infection should also be considered in patients who present with febrile illness and rash following a stay in a high-endemic country. The absence of macular exanthema, pharyngitis and lymphadenopathy weighed against such a diagnosis, nor did the patient report any sexual exposure. Dengue fever is also expected to present symptoms of macular exanthema as well as myalgia and thrombocytopenia (3). This diagnosis was therefore unlikely.
Trypanosomiasis (East African sleeping sickness) is extremely rare in Norway (4), and the patient could not remember having been bitten by tsetse flies. Nor were there any signs of primary skin rash, meningism or sensory effects. Protozoan diseases such as malaria and trypanosomiasis do not normally cause eosinophilia either (5). Our patient had slight eosinophilia. Acute schistosomiasis (Katayama fever) usually causes eosinophilia (6), but was considered unlikely because the patient reported no exposure to fresh water, the incubation period before the onset of fever is normally longer than in this case, and the eosinophilia is often more pronounced.
During the patient’s stay in hospital, no evidence was found of bacterial or viral infectious disease or affection of the central nervous system. His general condition and vital parameters were stable, while the urticaria came and went. A parasitic disease due to roundworm or flatworm (tapeworm or trematodes) with affection of the skin, lungs and possibly liver was strongly suspected.
Pneumonia due to multicellular parasites may be due to direct invasion through inhalation, haematogenic/lymphatic spreading or eosinophil accumulation in response to helminth infection in the incubation phase (Löffler’s syndrome) (7). In light of radiological findings, the latter was considered most likely in our patient. The most common causes of Löffler’s syndrome are roundworm infections due to the giant roundworm (Ascaris lumbricoides), Old World hookworm (Ancylostoma duodenale) and New World hookworm (Necator americanus) or Strongyloides stercoralis. Other roundworms may cause similar lung disease (visceral larva migrans, trichinosis, dirofiliariasis). Echinococcus is an example of a flatworm that can affect the lungs. Neither pulmonary cystic hydatidosis (E. granulosus) nor alveolar echinococcus (E. multilocularis) fit the incubation period or clinical and radiological findings for this patient. Paragonimus or schistosome species are the main trematodes that can cause lung affection. The former are transmitted by intake of raw shellfish, but our patient had not eaten sushi or any other seafood without adequate heat treatment during his stay in Africa.
Discussion
The combination of fever, cough, urticaria, periorbital oedema, diffuse, small-nodular lung opacities and eosinophilia following a period in Africa gave rise to suspicion that this patient was suffering from a parasitic disease. Eosinophilia and Löffler symptoms are not seen with malaria or other protozoan infections (5) and are caused by the helminth diseases in the incubation phase (7). It is important, nonetheless, to exclude serious febrile imported diseases such as falciparummalaria and typhoid fever, as eosinophilia can conceivably have other causes, such as an adverse reaction to a drug (e.g. malaria prophylaxis). However, exposure, the clinical picture and strong positive schistosoma serology indicated that the patient had Katayama fever, i.e. acute schistosomiasis.
Schistosomiasis, also known as bilharzia, is a tropical parasitic disease caused by a 10 mm long flatworm (trematode) of the Schistosoma family. Five species cause disease in humans: S. mansoni, S. japonicum, S. mekongi and S. intercalatum, which cause a hepatointestinal form of the disease, and S. haematobium, which causes urogenital disease. More than 207 million are estimated to be infected, of whom 85 % in Africa, where the disease burden is particularly high (8). The disease results in the loss of 70 million disease-adjusted life years, comparable to malaria, tuberculosis and HIV. At the same time, the disease is often described as neglected, as less than 5 % of those infected receive treatment (9).
The parasite has a complex life cycle (Fig. 1). Humans are the definitive host, and fresh-water snails are the intermediate host. Fertilised eggs are excreted with faeces or urine. In freshwater, the eggs mature into infectious larvae (cercaria) which penetrate the skin of the primary host and end up in the liver. After maturing in the liver, the larvae find their way to the venous plexa in the urinary tract and genitalia (S. haematobium) or to the gastrointestinal canal (S. mansoni and others). The adult male and female worms live together, reproduce and secrete fertilised eggs. These either end up in the environment or remain embedded in various tissues, where they induce a granulomatous inflammatory reaction. Over time, this results in fibrosis and organ damage, which is partly species-dependent (10).
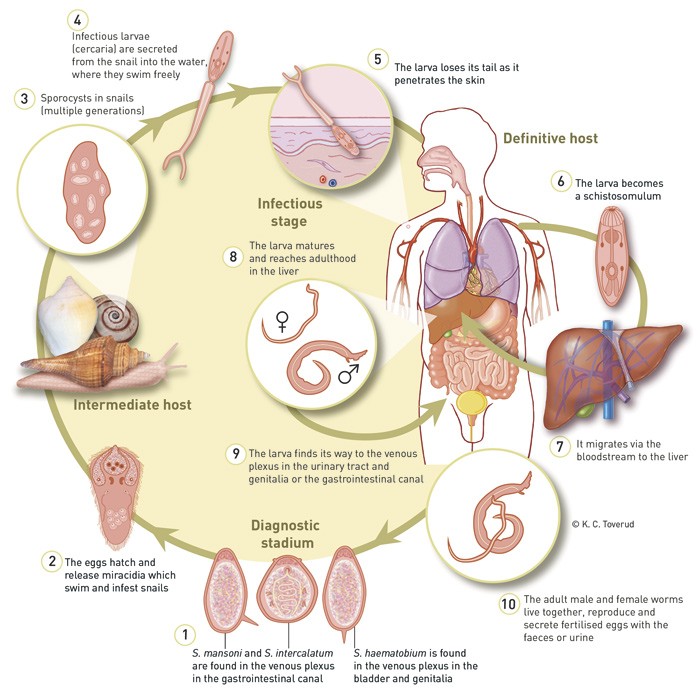
Figure 1 The life cycle of the schistosoma parasite
Schistosomiasis is endemic to the Middle East and Africa between the Sahara and South Africa, along the Nile and in Madagascar (S. haematobium, S. mansoni, S. intercalatum). In South America, S. mansoni is found along the coast of Brazil, while S. japonicum is found in China and the Philippines. As its name suggests, S. mekongi occurs widely in the Mekong basin (10 – 12).
Cercaria dermatitis is an acute, transient hypersensitivity dermatitis which occurs a short time after the cercaria have penetrated the skin. Our patient did not report any such symptoms.
In endemic regions, chronic schistosomiasis is the most widespread type of disease, and is due to repeated exposure to infectious cercaria. The first infection tends to occur at the age of two, with an increasing parasite burden through the next decade and subsequent decline in adulthood. In these areas, 60 – 80 % of school children and 20 – 40 % of adults may be infected (10). The chronic form of S. mansoni infection presents with non-specific symptoms such as abdominal pain, diarrhoea and rectal bleeding. Liver fibrosis is often periportal, and seldom results in liver failure. Portal hypertension, with splenomegaly and oesophageal varices, occurs, however. S. haematobium causes urogenital symptoms, with haematuria and dysuria and in many cases leads to hydronephrosis and kidney failure. The infection also increases the risk of carcinoma of the bladder, and both female and male genital schistosomiasis affect reproductive health. Genital lesions are assumed to explain an increased HIV transmission rate (8, 10).
Katamaya fever usually affects non-immune subjects who are exposed for the first time and at a higher age than the natives in endemic regions (6, 11). The condition usually occurs weeks to months after primary infection, and is a consequence of worm maturation and egg production. This releases antigens into the circulation, which strongly stimulates the immune system. The typical clinical presentation of Katamaya fever is acute onset with fever, malaise, fatigue, myalgia, headache, abdominal pain and eosinophilia. Fever onset is 3 – 8 weeks after exposure, while urticaria may occur somewhat earlier. Cough, abdominal pain and eosinophilia usually occur after the onset of fever. Seroconversion tends not to occur until six weeks after exposure, while secretion of eggs into faeces or urine starts even later (10, 11).
Lung affection in connection with acute schistosomiasis was first reported 60 years ago. Symptoms such as cough, laboured breathing and chest pain have been described for all varieties, but are most common with S. mansoni infection (13). The pathophysiology is only partially understood, but the impact of immune complexes, eosinophil infiltration and release of proinflammatory cytokines probably plays a part. Affection of the central nervous system is rare, but more serious. For example, transverse myelitis may occur, as a result of ectopic egg deposition and inflammation (10, 13).
The diagnosis of schistosomiasis depends on the disease stage and parasite burden. Microscopy of concentrated urine and faeces, with identification of characteristic eggs, is still the gold standard for diagnosis of active parasitic disease (12). This applies particularly to persons with a chronic infection. In non-immune travellers, and particularly in cases of Katayama fever, the parasite burden is often so low that microscopy yields negative findings (11). Malaria prophylaxis with mefloquine may also reduce egg production (11, 14).
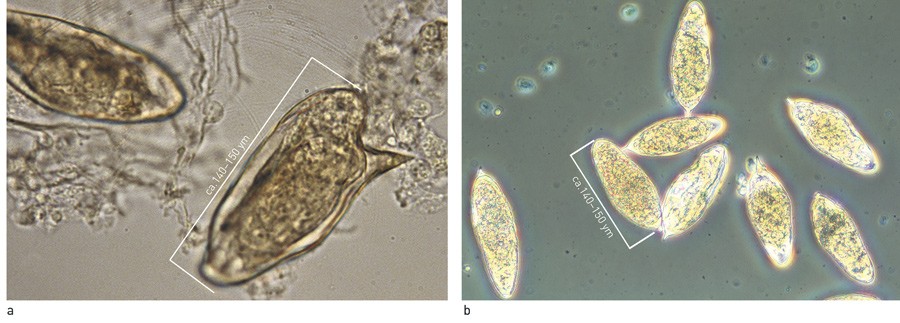
Figure 2 Eggs of a) S. mansoni and b) S. haematobium
An early, low-grade infection is mainly detected through antibody tests, which are more sensitive than microscopy (6). Serological tests do not distinguish between active infection and past exposure (10, 13), and with immigrants from endemic countries, this may present a diagnostic challenge. However, falling antibody titres can be expected immediately after treatment, and treatment will usually be offered to persons from endemic areas with positive serology unless information indicates that they have received treatment recently. In ethnic Norwegian travellers, however, background immunity is negligible. Most routine techniques detect IgG, IgM or IgE against soluble worm and egg antigens with ELISA, immune haemagglutinine or immunofluorescence analyses. Seroconversion usually occurs 4 – 8 weeks after infestation, sometimes longer, and usually after the onset of symptoms. Our patient had a positive serostatus six weeks after exposure. A combination of several tests is used to increase sensitivity and specificity because of cross-reactivity between schistosoma species. Monoclonal antibody tests and Western blot have also been used to achieve higher specificity (11). As with our patient, species determination must also be considered in light of the clinical picture, incubation period, epidemiology and the patient’s travel history, and the infection has very probably been caused by S. mansoni. Antigen tests are also commercially available (15), but are not used much in Norway because of the rarity of the condition.
In the future, detection of schistosoma DNA by means of molecular genetic methodology may to some extent replace microscopy diagnostics, but at present it is not readily available for routine diagnostics.
Schistosomiasis treatment has three main aims: to reverse acute and early chronic disease; to prevent complications of chronic infection; and to prevent rare, severe complications such as neuroschistosomiasis. Praziquantel represents first-line treatment, and is active in relation to all species (10 – 12). The mechanism is not fully understood, but the treatment efficacy presupposes a functioning immune system. The drug has no effect at all on eggs and limited effect on immature larvae. Treatment can therefore usually be postponed until about six weeks after exposure. Alternatively, re-treatment can be conducted 4 – 6 weeks after primary treatment, in order to capture maturing larvae (11). In Norway, Praziquantel can be procured with exemption from registration as 600 mg tablets. The standard dose is 40 mg/kg for S. mansoni and S. haematobium, consisting of 1 – 2 doses the same day.
At present artemisinine derivatives are first-line therapy for severe malaria, but the drugs have also proved effective against schistosoma larvae. There are indications that combination therapy increases the therapeutic efficacy compared with praziquantel alone (12, 16).
Steroids can be administered in cases of Katamaya fever to dampen the hypersensitivity reaction (12), but the optimal duration of therapy has not been investigated in controlled trials.
