A man of African origin was hospitalised with headache and backache. MRI yielded surprising findings. Imported diseases must not be forgotten, even in persons who have lived in Norway for a long time.
A man in his forties from an African nation was admitted to the medical department of a Norwegian local hospital with headache and backache. He had lived in Europe for many years without visiting his native country. He had previously had malaria and iridocyclitis but had otherwise been mostly in good health. However, for four years prior to his current hospitalisation, he had been suffering from right-sided back pain, although this had not required him to give up work. A lumbosacral spine CT scan from that time showed no signs of pathology. One month prior to the hospitalisation, the patient had undergone MRI of his right ankle because of pain, but the results had been normal.
Upon admission, he complained of a global headache that had been present for six days. He also noted somewhat impaired vision and pain upon exposure to strong light. He felt pain in his entire body and weakness in all of his muscles. The lumbar pain had worsened. For the last four days he had had dysuria and difficulty urinating. Prior to transfer to the hospital, he had been catheterised and had 500 ml of urine drained in the Accident and Emergency department. Urine dipsticks were negative.
Upon admission the patient was afebrile and an initial neurological workup was normal. All routine blood tests with respect to haematology, infection, renal function and liver function were also normal. The following day the patient still had headache, as well as pain throughout his entire body. He had difficultly lifting his right thigh when walking, but could otherwise walk normally. We considered the headache to be the patient’s main problem.
The man spoke good Norwegian but in hindsight we see that communication could have been better. Both patient and doctors focused on the new-onset headache, with other symptoms regarded as secondary to this. The patient had normal reflexes in the lower limbs and a flexor plantar reflex. We considered there to be no evidence for a cross-sectional lesion and regarded the difficulty with urination as pain-related. He was referred for MRI of the head, which was described as normal. CT scanning was not available at that particular point in time.
That same evening the patient was re-examined by another doctor on duty. He ordered an MRI of the lumbosacral spine owing to the continued urinary retention and back pain.
Spine MRI showed a pathological intramedullary lesion at level Th10/Th12 (Fig. 1a, b). In the distal medulla, a 6-cm-long spindle-shaped expansion was seen that extended to the conus. The lesion had an inhomogeneous signal, higher than that of the medulla on T2-weighted images. Use of contrast revealed patchy, inhomogeneous contrast enhancement. The medulla was so enlarged that it filled most of the spinal canal.
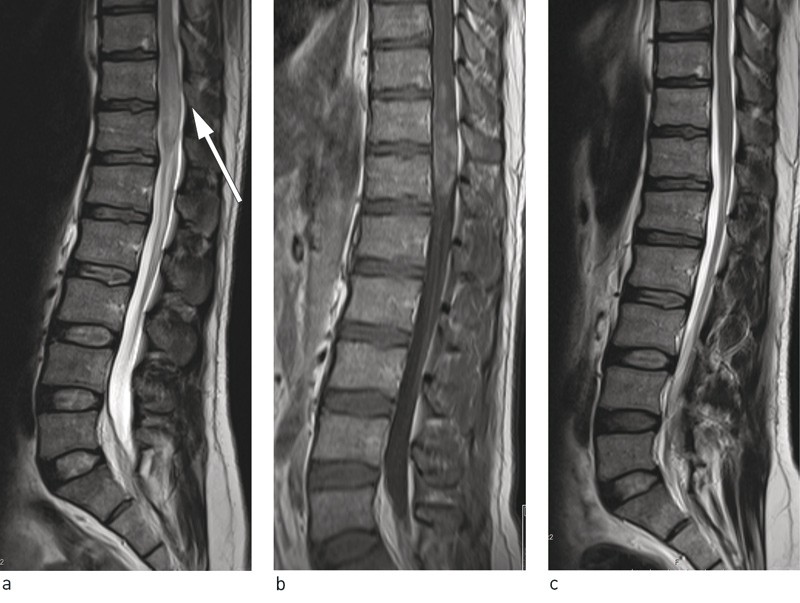
Figure 1 Lumbosacral spine MRI showing expansion of the medullary cone at level Th 11/Th 12 with hyperintensity on T2-weighted sequences and contrast enhancement on T1-weighted sequences. a) Sagittal T2-weighted sequence prior to treatment, b) Sagittal T1-weighted sequence with intravenous contrast prior to treatment and c) Sagittal T2-weighted sequence post-treatment
The findings were not typical of a solid tumour, transverse myelitis or demyelinating disease, although these could not be ruled out. On the basis of the images and clinical examination, we considered an infectious aetiology to be a highly relevant differential diagnosis, and the examining radiologist noted that schistosomiasis could have a very similar appearance. Tuberculosis was less likely because of the localisation and appearance of the lesion and because isolated involvement of the medulla is very rare.
On suspicion of schistosomiasis in the spinal canal, the patient was transferred to the nearest university hospital with expertise in infectious diseases and neurosurgery. No indication was found for surgical intervention. Lumbar puncture revealed 415 leukocytes in the cerebrospinal fluid (CSF) (ref < 5/mm3). CSF protein was elevated to 624 (ref. 100 – 500 mg/l), CSF albumin 372 (ref. 100 – 300 mg/l), CSF glucose 4.1 mmol/l (ref. dependent on blood glucose). Immunophenotyping of the cerebrospinal fluid provided no evidence of lymphoma.
The results of the cerebrospinal fluid analysis with high cell count and protein levels, combined with the MRI findings, suggested an inflammatory disorder of the central nervous system. No parasites were detected upon microscopy of urine and faeces, and a spinal canal mycobacterial culture was negative. Negative results were also obtained for Mycobacterium tuberculosis complex DNA, serum-TB IGRA Quantiferon, hepatitis B and C and serum Cysticercus Western blot antibody (sample sent to Basel).
Samples sent to the Public Health Agency of Sweden, based in Stockholm, for testing for schistosomiasis showed the following: serum ELISA egg titer 22 (threshold 10), serum Schistosoma immunofluorescence negative, and serum Schistosoma immunofluorescence gut-associated antigen positive 270 (threshold 10). The ELISA egg titer and antigen results suggested schistosomiasis infection.
Treatment was initiated with methylprednisolone 16 mg × 1 orally, increasing to 16 mg × 4 orally the next day. The dose was reduced after 11 days and subsequently tapered over five weeks. He was also given praziquantel 1 500 mg × 2 orally for three days, and this was repeated 14 days later. He had urinary retention for the first week necessitating catheterisation, and experienced transient problems with urinary retention following catheter discontinuation. The patient’s weakness in the lower extremities improved. He was transferred back to the local hospital after three weeks, and was followed up by the hospital’s rehabilitation team after discharge.
Upon examination one year after discharge, he continued to have right-sided lumbar pain but this was less severe than prior to treatment for schistosomiasis. The patient stated that he had had problems with urination for the first three months, but that these had gradually resolved. He had no paresis. He described mild hypoesthesia in his lower right limb and mildly impaired vibration sense. The man was in occupational training and wished to return to work. An MRI showed complete regression of the previously observed lesion (Fig. 1c).
Discussion
Schistosomiasis is an infectious disease caused by blood flukes, also known as trematodes. More than 230 million people are estimated to be infected, although the true figure is probably much higher (1). In humans, schistosomes exist as 1 – 2 cm long worm-like parasites in perivascular locations within the venous system or in the mesentery, but may also be found elsewhere. Male and female flukes live paired together and can move within the vascular tree. The flukes can live for up to 40 years, but their normal lifespan does not exceed 3 – 10 years (1).
Each pair lays thousands of eggs that are excreted via the urinary bladder or the intestines. Eggs can remain lodged in tissue and cause significant problems for the infected host. The tissue may react against the eggs, with a subsequent humoral and cell-mediated response generating granulomas many times larger than the egg itself.
The prevalence of schistosomiasis is highest in sub-Saharan Africa. The disease also occurs in South America and Asia, and a total of five different species cause disease in humans (1).
Fresh water is essential for the parasite to complete its life cycle. The eggs are excreted into the water via the host’s urine or faeces. Once there, the parasite continues its development using a snail as an intermediate host, and releases a cercaria which can infect humans upon contact with the skin. The cercaria penetrates the skin and moves by passive intravascular migration via the lungs to the liver. After 1 – 3 months, the parasite will have developed into an adult fluke. Male and female flukes form a pair and move to the species’ preferred final niche (Fig. 2). For Schistosoma mansoni and Schistosoma japonicum, this is the inferior mesenteric veins and colon, whereas Schistosoma haematiobium makes its way to veins surrounding the urinary bladder. The flukes can also move to other organs, however.
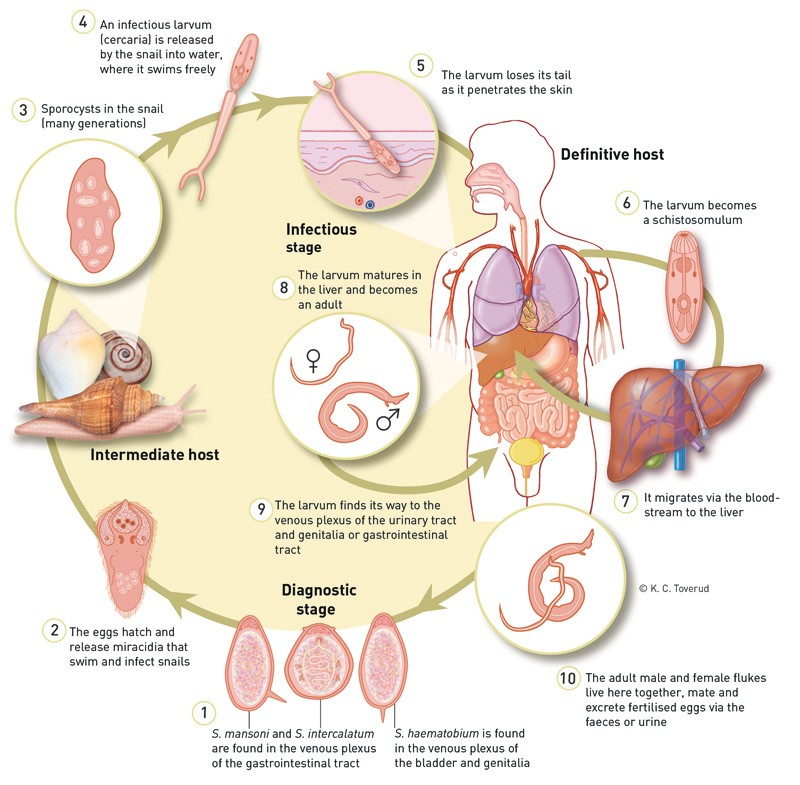
Figure 2 The life cycle of the Schistosoma parasite
Ectopic flukes are most often found in the central nervous system, in the perivertebral venous plexus or cortical cerebral veins. Egg deposition in the central nervous system may trigger a cell-mediated periocular granulomatous reaction, leading to neurological complications. The mass effect of tens of thousands of eggs plus the large granulomas in the brain or spinal cord can account for symptoms such as increased intracranial pressure, myelopathy, radiculopathy and subsequent sequelae. Myelopathy in the lumbosacral region is the most common complication of S. mansoni and S. haematobium infection, whereas acute encephalitis in the cortex, subcortical white matter, basal ganglia or internal capsule is typical for S. japonicum (2, 3).
Neurological complications have been described in numerous case reports with small numbers of patients. The majority describe patients from endemic regions, but there is also a report of a tourist who developed symptoms four years after bathing in fresh water while travelling in West Africa (4).
Neuroschistosomiasis is a consequence of central nervous system involvement in schistosome infection and requires prompt treatment if symptoms emerge. Complete remission of symptoms after treatment has been described in several case reports (5 – 7). There are three main forms of the condition. One is acute schistosomal encephalopathy, for which the direct aetiology remains unknown. Headache, altered sensation, seizures, ataxia and cerebellar symptoms are seen most frequently (2).
A granulomatous reaction in brain tissue may give rise to a pseudotumour leading to increased intracranial pressure. Headache, visual disturbances, seizures and altered mental status are the main symptoms.
A granulomatous reaction in the spinal cord is the earliest described and best known form of neuroschistosomiasis. Patients often have no other symptoms, but symptoms in such cases may also vary greatly. Back pain is often the first symptom with radiating pain in the lower extremities. Others may present with weakness of the lower extremity muscles, bowel and urinary bladder dysfunction, paraesthesia, impotence in men and altered reflexes in the lower extremities (2).
Direct diagnostic methods, which aim to detect living eggs, are the only way to unequivocally confirm an ongoing schistosomiasis infection. The various Schistosoma species that have been identified have different eggs. Microscopy of normal and filtered urine is used in areas where schistosomiasis is widespread. Direct microscopy of stools has insufficient sensitivity, although this can be increased via special concentration techniques. The presence of eggs in a biopsy can also confirm the diagnosis (1).
There are a number of immunological tests that detect circulating anti-schistosomal antibodies. However, these reveal nothing about the degree of infection, do not differentiate between previous and current infection, and are not species-specific. The cost of these tests and a lack of technology means that they are not available to the most relevant patients in countries with the highest disease burden. These tests are not performed in Norway either; samples are sent to a laboratory in Sweden. The Public Health Agency of Sweden conducts tests using immunofluorescence against gut-associated antigen (GAA) and somatic antigen (SA). If at least one of these two is positive, ELISA is used to test for soluble egg antigen (SEA). Gut-associated antigen comes from the parasite’s gut and is regurgitated by the fluke after it has digested a blood meal. It is typically the first test to show a positive result after infection, and may be positive even before eggs can be detected in the urine or faeces. Soluble egg antigen, as the name suggests, shows positive only once egg production has started. Somatic antigen is a bodily antigen from the fluke itself and is typically seen in chronic infections where some flukes have begun to die, or following treatment (senior consultant Tore Lier, personal communication).
Assessment of schistosomiasis infection in hepatosplenic and urogenital organs can be performed using ultrasound, CT and MRI. Ultrasound can be used at the bedside and in the field and requires significantly less financial investment than the other modalities (8). CT scanning is particularly good for showing calcification of the urinary tract, which is typical of urogenital schistosomiasis (9). For ectopic infection with cerebral involvement or myeloradiculopathy, MRI is the optimal method. However, this modality is rarely available among the populations that are most at risk. In cases of neuroschistosomiasis, the distal medulla/conus is most frequently affected. On T2-weighted sequences, heterogeneous hyperintensities are seen along with expansion of the medulla, often over several segments. The presence of eggs and consequent granuloma formation may result in nodular contrast enhancement in the medulla and peripheral enhancement in the leptomeninges. In a number of cases there is also involvement of nerve roots and the cauda equina (10 – 12).
Treatment is directed chiefly at eliminating the flukes. Treatment of immunopathological complications caused by the eggs may also be appropriate.
Praziquantel in a single dose of 40 mg/kg is sufficient for all Schistosoma species. No serious side effects have been reported.
In cases of neuroschistosomiasis, treatment with praziquantel may lead to exacerbation of the immunological response. Treatment with steroids is therefore recommended before the elimination of flukes.
Our patient had spent at least 11 years in countries free of schistosomiasis prior to being hospitalised. That flukes can survive for many years in the human body highlights the need to keep imported diseases in mind, even for persons who have been in Norway for a long time. Myelopathy due to schistosomiasis has been described in one patient 22 years after they were last exposed to infected water (13).
Myeloradiculopathy caused by schistosomiasis is a serious and under-recognised complication (6). The prevalence of this condition in centres in Brazil and Africa that handle cases of non-traumatic myelopathy is estimated to be 1 – 5 % (2, 14).
Cerebrospinal fluid analysis shows increased protein concentration and mononuclear cell count in 90 % of patients with such myeloradiculopathy (14), and this was also true for our patient. MRI-based diagnosis has proven particularly valuable for this patient population and led to the correct diagnosis in our case too.
Upon examination one year post-treatment, the MRI findings in the spinal canal had completely resolved. This shows that treatment is still effective, even long after the time of infection. This observation is important because very few persons with neuroschistosomiasis are examined using MRI.
