A woman in her thirties was acutely hospitalised with dyspnoea and chest pain. The cause of the symptoms would prove to be an unusual manifestation of a rare disease.
A woman in her early thirties, with a history of mostly good health, was admitted acutely to the cardiology department with a diagnosis of chest pain. She had recently experienced gradually increasing functional dyspnoea.
In the week prior to hospitalisation, she had had respiratory chest pain with radiation to the throat and jaw. She had also had intermittent fever on a number of occasions in recent weeks. She had had a spontaneous abortion about six weeks earlier, after 8 – 9 weeks of pregnancy.
The patient’s medical history, age and risk factor profile gave little reason to suspect coronary artery disease as a cause of the symptoms. In the age group to which the woman belonged, other causes of chest pain are more likely, such as pulmonary embolism, pleuritis, pneumothorax, pericarditis and gastroesophageal reflux.
Upon admission, vital signs were as follows: pulse 106/min, regular, blood pressure 110/77 mm Hg, temperature 37.0 °C, respiratory rate 16/min. Auscultation of the heart revealed a murmur over the entire precordium. Palpation of the abdomen revealed a firm mass in the lower right quadrant that was sore upon direct palpation.
An ECG revealed sinus tachycardia, but no definitive pathology. Blood tests showed a moderately elevated CRP level of 59 mg/l (<5 mg/l) and elevated D-dimer of 1.80 mg/l (<0.50 mg/l), troponin T 66 pmol/l (< 15 pmol/l) and proBNP 244 pmol/l (< 33 pmol/l).
A heart murmur indicates turbulence in the normal blood flow. Turbulence may occur as a result of damage to the heart valves or may reflect anatomical defects in the heart, a high flow rate or reduced blood viscosity.
Upon discovery of the murmur, a preliminary echocardiographic examination was performed in Accident and Emergency. This revealed two large masses on the right side of the heart. The largest mass measured 3.5 x 3 cm, was located within the atrium and adherent to the tricuspid valve apparatus. In addition, there was a smaller mass that also penetrated the right ventricle.
There was clear obstruction of normal blood flow in the tricuspid ostium. Pericardial effusion was also seen, particularly along the right ventricle, with a maximum size of 1.5 cm but no evidence of tamponade. The mitral and aortic valves on the left side of the heart appeared normal. The findings were confirmed with a cardiac CT scan, which also raised suspicion of infiltration of tumour masses into the right ventricular wall and pericardium (Fig. 1).
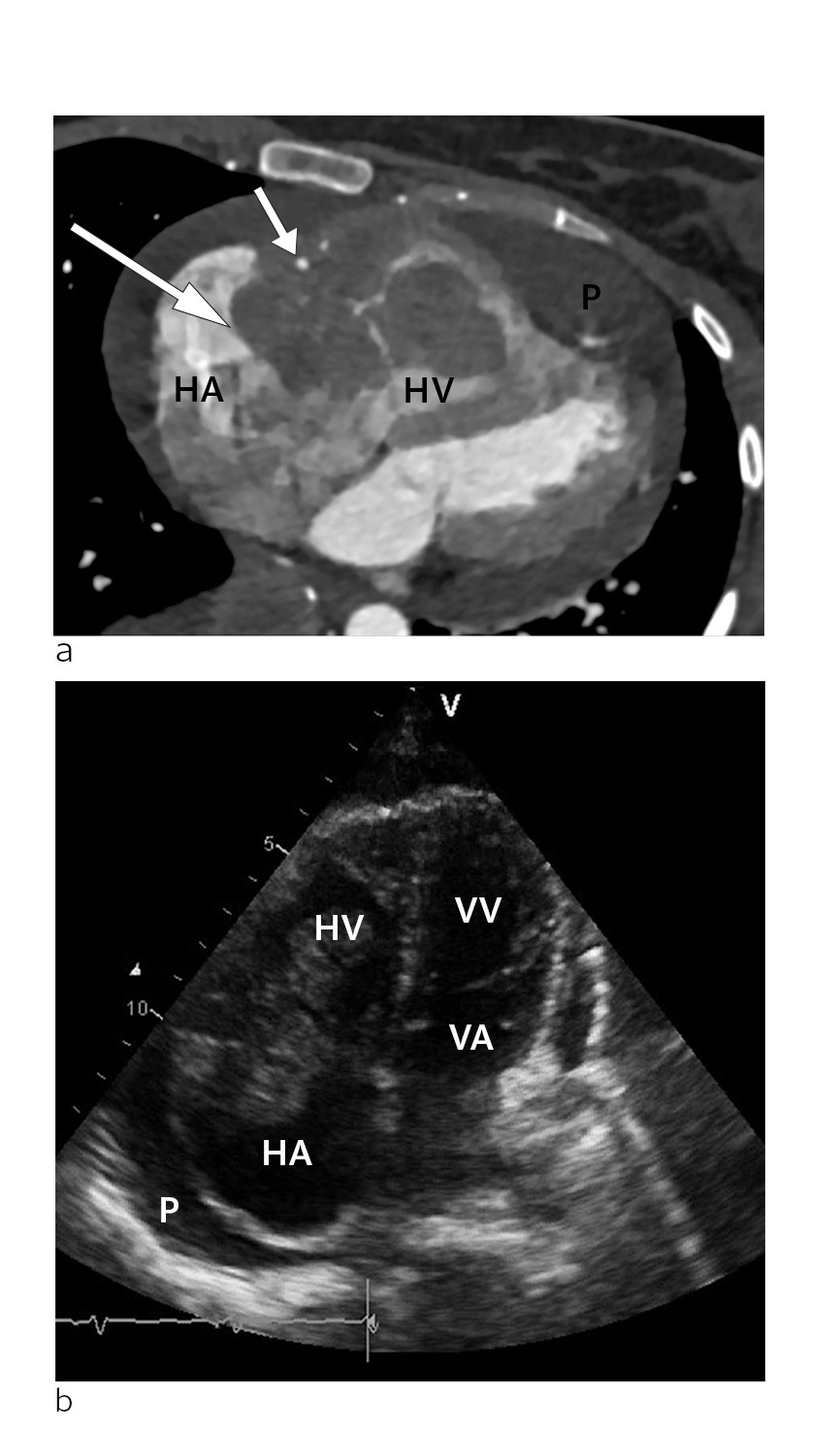
Figure 1 a) CT scan of the heart in the transverse plane at the time of diagnosis. Tumour (large arrow) in the right atrioventricular groove, surrounding an open right coronary artery (small arrow). The mass is located within the right atrium (HA) and right ventricle (HV). The tumour is also infiltrating the free wall of the right ventricle (HV). Pericardial effusion (P) can be seen around the heart, and is most pronounced apically. B) Echocardiogram at the time of diagnosis. Apical four chamber view showing the right atrium (HA), right ventricle (HV), left atrium (VA) and left ventricle (VV). The tumour is located at the transition between the right atrium and right ventricle, where it almost completely occludes the tricuspid ostium. Pericardial effusion (P) can be seen around the heart.
Cardiac masses are relatively rare. They may be classified as neoplastic or non-neoplastic (1). The latter include thrombi, pericardial cysts, valvular vegetations and prominent anatomical structures such as lipomatous hypertrophy of the interatrial septum.
Neoplastic masses may be primary benign, primary malignant or metastatic. Myxomas are the most common form of primary benign tumours. Malignant conditions are rare.
The patient’s symptoms of chest pain and dyspnoea and the clinical finding of murmur were judged to be related to a cardiac tumour, with a primary tricuspid valve obstruction. The pericardial effusion was considered to be of little haemodynamic significance and we therefore decided against drainage.
It can be difficult to distinguish tumours from other masses such as vegetations or thrombotic masses. For this reason, we chose to treat the patient both for primary cardiogenic thrombus, with low molecular weight heparin, and as for endocarditis, with broad spectrum antibiotics in the form of penicillin and gentamycin.
Further diagnostic imaging was performed, including CT thorax, abdomen and pelvis. This verified the echocardiographic finding of an expansive process in the right side of the heart with pericardial effusion. An additional large expansive process measuring 11 x 8 cm was found in the right side of the abdomen, with varying density and contrast enhancement, calcifications and abundant vascularisation. The pouch of Douglas was also shown to contain free fluid and masses with minor contrast enhancement (Fig. 2).
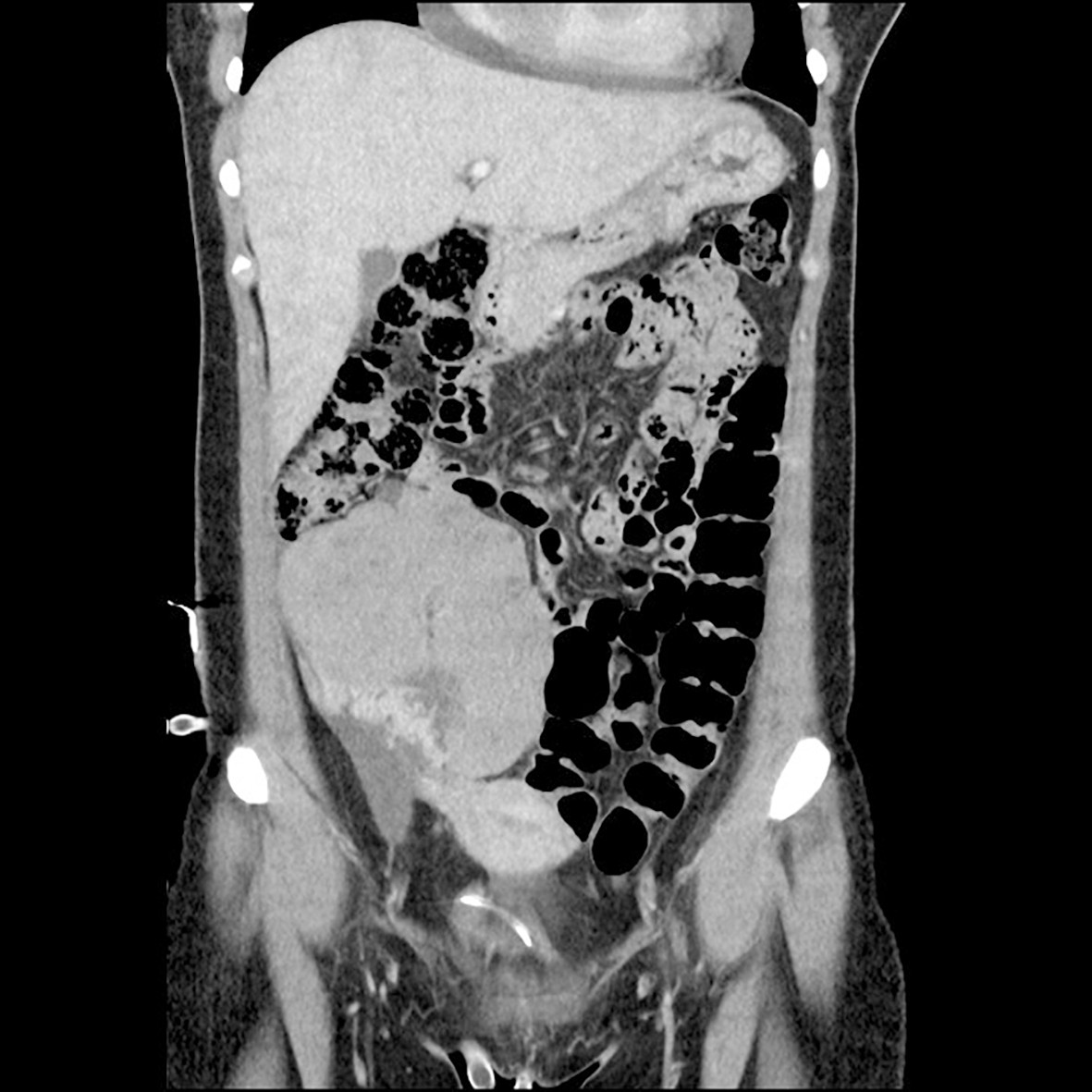
Figure 2 CT abdomen in the frontal plane at the time of diagnosis. A CT scan of the patient after hospitalisation revealed a large mass of 11 x 8 cm in diameter in the lower right quadrant of the abdomen. The mass was of varying density and contrast enhancement, and showed calcifications and abundant vascularisation.
Two distinct abnormalities were thus detected in the patient: a cardiac mass and a tumour in the abdomen/pelvis. A key next step in the workup was to determine whether these were two separate findings or whether they were related. An interdisciplinary approach is important in such cases.
An interdisciplinary discussion took place between cardiologists, thoracic surgeons, oncologists, gynaecologists and gastrointestinal surgeons. Intervention with respect to the cardiac abnormalities was considered to be too risky. Acute cardiac surgery was seen as a last resort, but we continuously monitored vital indication for surgery.
The pathology in the abdomen and pelvis close to the right ovary was perceived as relatively accessible for biopsy. The biopsy was performed under ultrasound guidance, and the sample was prepared as frozen sections with rapid results a priority. After collection of the sample, the patient was placed on high dose steroids in the form of dexamethasone 3 mg x 3.
Patients with an unclear diagnosis involving multiple organ systems often require a broad approach that includes multiple specialists. The risks and benefits of various diagnostic and therapeutic interventions must be considered carefully. For the vast majority of masses of unknown origin, tissue histology will provide diagnostic clarification and form the basis for further management of the condition.
The day after the biopsy, preliminary results from the pathology department were available: high grade malignant lymphoma. As the lymphoma was considered potentially life-threatening, direct anti-tumour therapy was started that same day with cyclophosphamide and dexamethasone at an increased dose of 4 mg x 4. Allopurinol and intravenous fluids were given as tumour lysis prophylaxis.
Only hours after treatment, the patient noticed decreasing chest pain and reduced dyspnoea. Two days later, it was possible to perform cardiac MRI. This confirmed persistent tumours in the right atrium and right ventricle with growth through the ventricular wall and fusion of the tricuspid leaflet (Fig. 3).
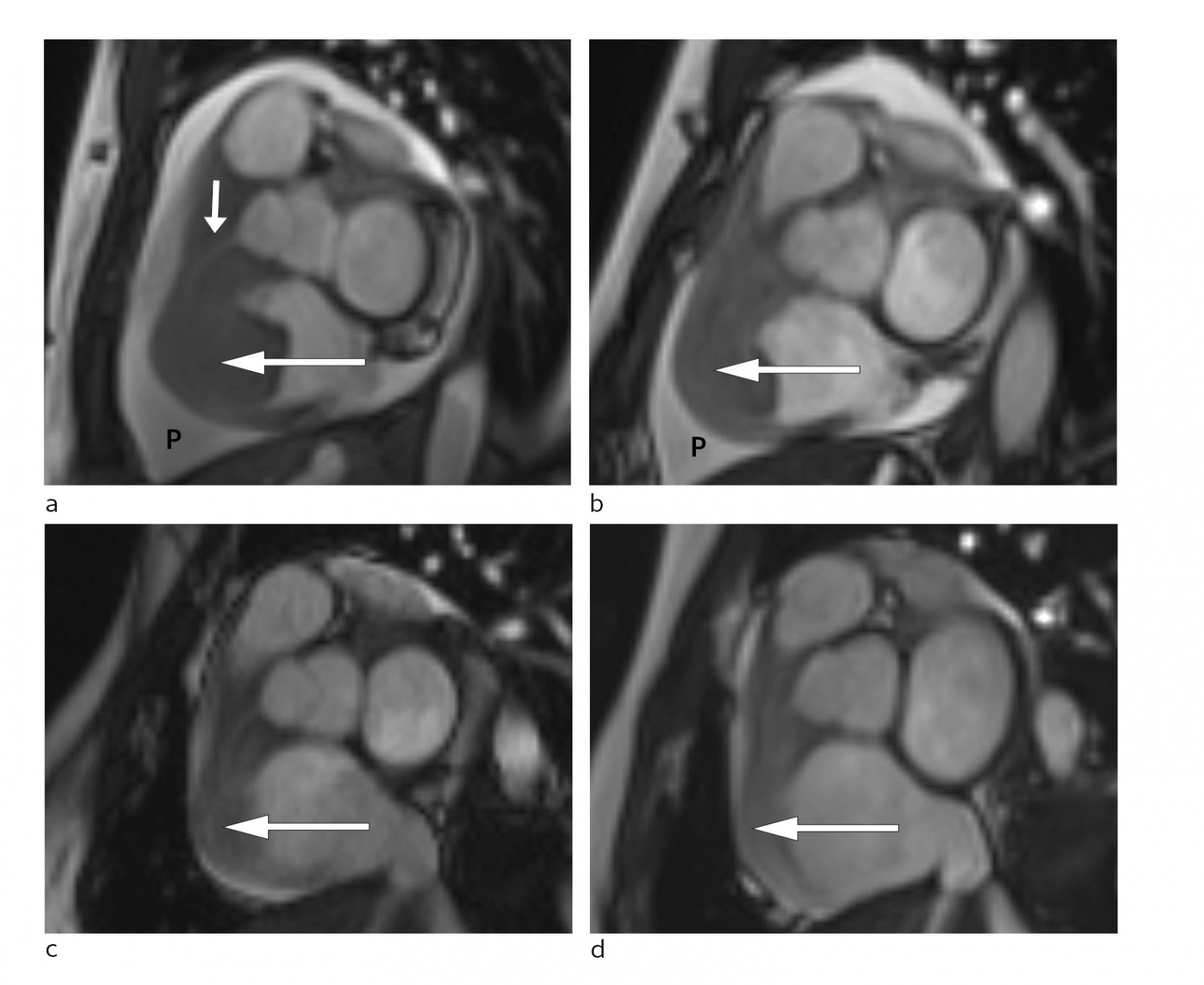
Figure 3 Short axis view of cardiac MRI at different disease stages. a) Large tumour at the time of diagnosis (large arrow) in the right side of the heart and significant pericardial effusion (P). The right coronary artery (small arrow) passes through the tumour. Follow-up MRI showing gradual reduction of tumour size (arrow) and pericardial effusion (P) at b) one week post-diagnosis, c) eight weeks post-diagnosis and d) 20 weeks post-diagnosis.
Disseminated malignancies and lymphomas can in very rare cases affect the heart, most often in the form of pericardial effusion. Intracardiac masses as part of lymphoma are very rare (2).
Even though the cardiac mass in our patient was not biopsied directly, it was considered highly likely to be a manifestation of the lymphoma that was detected. A clinical and radiological therapeutic response would support such a conclusion.
A few days later, the final results became available from the pathology department. The conclusion was that the patient had a high grade malignant lymphoma, morphologically and immunophenotypically consistent with diffuse large B-cell lymphoma of the germinal centre subtype (positive for B-cell markers and BCL6, negative for BCL2, MYC, CD10 and MUM1). There was evidence of very high cell turnover: the Ki-67 proliferative index was almost 100 % (Fig. 4).
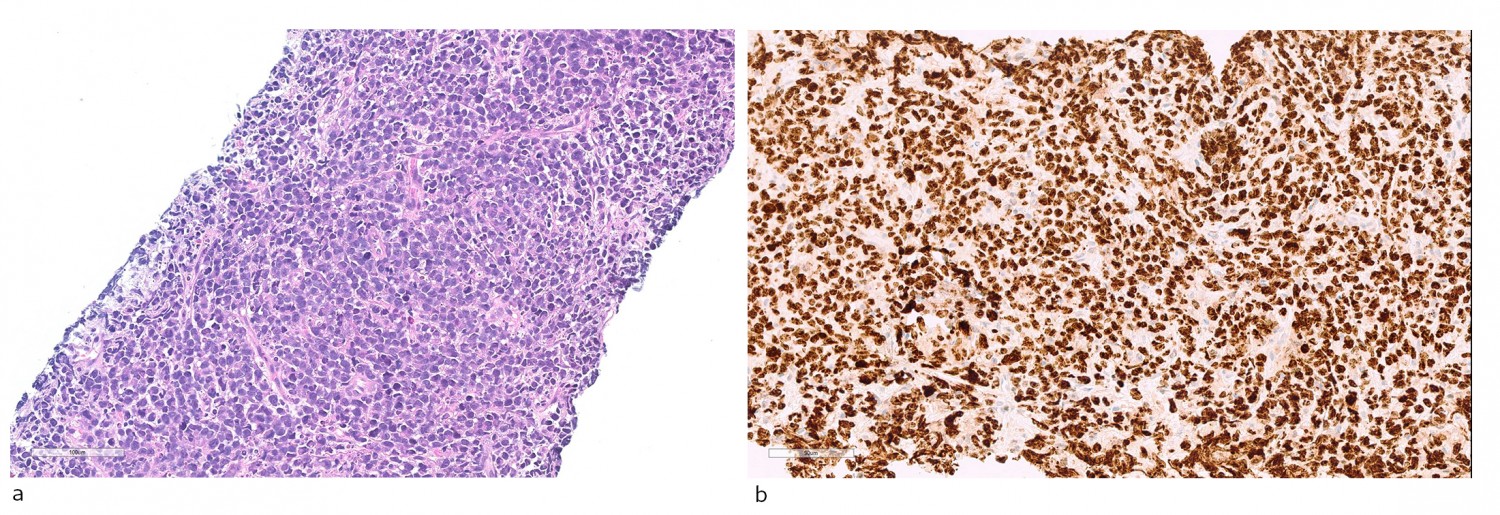
Figure 4 Histopathological diagnosis. a) Haematoxylin-eosin stained sections with diffuse infiltration of moderately large lymphoid cells with blast cell characteristics, with many mitoses and apoptotic bodies. B) Immunoperoxidase staining for the proliferation marker Ki-67 shows a high degree of positivity
The disease was detected in the right side of the heart, bilaterally in the kidneys and as a tumour in the abdomen and pelvis. All manifestations were extranodal, and the lymphoma was thus classified as stage IV. After initial treatment, we continued with the first main treatment, namely the standard chemotherapy regimen for non-Hodgkin’s lymphoma, CHOP: cyclophosphamide, vincristine, doxorubicin and prednisolone.
During this period, the patient was monitored in the cardiac observation unit. No arrhythmias or other complications of treatment were observed. She received low dose fractionated heparin as thrombosis prophylaxis. Repeated MRI scans, performed three days after initiation of the CHOP regimen, showed significant regression of the mass in the right side of the heart and almost total regression of the intracavitary components. The right atrioventricular opening was significantly larger than in the initial MRI scan (Fig. 3).
Rapid treatment initiation is indicated in cases of newly diagnosed high grade malignant lymphoma (3). The CHOP regimen is well established and has been the mainstay of treatment for non-Hodgkin’s lymphoma for the past 20 years. The monoclonal CD20 antibody rituximab is often used in addition: the so-called R-CHOP regimen (3).
We decided not to use rituximab therapy in the initial treatment of this patient. Radiological remission is the main indicator that a malignant disease is chemosensitive.
The patient responded well to the initial treatment. However, the condition was considered to be high-risk disease owing to the extranodal involvement, therefore we decided to provide further cycles of treatment. The first cycle was performed 25 days after the initial hospitalisation. There were no notable complications, and she was discharged to her home following post-treatment observation. Forty days had passed since she was first hospitalised.
The patient underwent a further treatment cycle. Radiological assessment after the second treatment showed almost total regression of the abdominal tumour (Fig. 3). We have continued with the R-CHOP regimen, which has kept the disease in remission ever since.
Discussion
Clinical examination of the patient led to the discovery of a heart murmur upon auscultation, and a palpable abdominal mass: both important findings that formed the basis for subsequent diagnostics. Echocardiography is a non-invasive procedure that in skilled hands can provide a great deal of information about cardiac function. Estimating the pump function of the heart and evaluating the status of the valve apparatus are the two most important matters.
Examination of the patient revealed a cardiac mass with a significant narrowing of the tricuspid ostium, which largely explains why she had become so short of breath on the slightest exertion. Cardiac masses are rare (1). The main causes of cardiac masses are summarised in Table 1. Biopsy is the key to further diagnosis, but represents an invasive procedure with a not insignificant morbidity and mortality risk.
Table 1
Classification of cardiac masses, modified from Kassop et al (1)
|
Non-neoplastic abnormalities
|
Benign neoplasms
|
Malignant neoplasms
|
|
Lipomatous hypertrophy
Intracardiac thrombi
Pericardial cysts
Large coronary artery aneurysms
Valvular vegetations
|
Myxoma
Lipoma
Fibroma
Haemangioma
Teratoma
Rhabdomyoma
|
Angiosarcoma
Rhabdomyosarcoma
Fibrosarcoma
Liposarcoma
Lymphoma
Metastasis
|
The patient was also found to have an abdominal mass, and although it was not possible to establish a definitive connection between these two findings, it made sense to perform the initial biopsy here as this was technically more straightforward. Rapid pathological analysis is important in such cases to allow prompt initiation of targeted treatment; in our patient this was performed on the basis of potentially vital indication.
Corticosteroid therapy was started before the final histology results were available. Corticosteroids have a general anti-inflammatory effect, in addition to a direct cytotoxic effect on lymphoid cells. Once the histology results were available, it was possible to begin a more targeted treatment of the cancer. It was important in this case to monitor the treatment response of both the primary abdominal tumour and the cardiac mass because a causal relationship between them had not been definitively established. The observed response (Fig. 3) was consistent with such a relationship.
While cardiac involvement is rare in lymphoma, it does occur, and then usually in the form of pericardial effusion (4, 5). Intracardiac masses as part of lymphoma are even more unusual, but may occur in disseminated disease (6); cardiac involvement is seen in 10 – 15 % of cases of advanced disease upon postmortem (7, 8). The right side of the heart is overrepresented in such cases, and the atria are overrepresented compared with the ventricles (6, 9). Multiple chambers are usually involved, as in our patient (10, 11).
Haematogenous spread is probably the most common cause of cardiac involvement. Thrombophilia with intracardiac thrombosis and secondary embolisation is not uncommon (12). The more malignant lymphomas, such as diffuse large B-cell lymphoma, Burkitt’s lymphoma and some forms of T-cell lymphoma, appear to be overrepresented in cases of cardiac involvement (6, 11). Comorbidity in the form of immunosuppression and immunodeficiency, including HIV infection, increases the risk of cardiac affection in lymphoproliferative disorders (5, 6, 13).
Treatment of malignant processes involving the heart can be challenging with a not insignificant risk of complications. Fatal outcomes, including myocardial perforation, have been described, and the diagnosis is often first established upon autopsy (6, 9, 14). Treatment modalities include chemotherapy, radiotherapy and surgery.
Lymphomas are generally chemosensitive tumours, and chemotherapy is usually the first-line treatment, including in cases with cardiac involvement (9). In our patient, treatment was initiated with steroids in the form of dexamethasone and alkylating chemotherapy in the form of cyclophosphamide. Both steroids and cyclophosphamide have documented antineoplastic efficacy in lymphoproliferative disorders and are the cornerstones of lymphoma treatment (3).
This case history illustrates the importance of an interdisciplinary approach and rapid diagnosis of a rare disorder for allowing, as in this case, treatment with curative intent.
