A healthy pregnant woman was monitored during pregnancy owing to polyhydramnios and suspected small intestinal atresia. In week 31 she attended the maternity unit with contractions and bloody discharge. There turned out to be a serious umbilical cord complication.
A woman in her thirties had given birth previously to two children: a healthy girl and a boy who was born with isolated intestinal stenosis. She was therefore monitored closely during her third pregnancy, and an ultrasound examination raised suspicion of small intestinal stenosis/atresia with development of polyhydramnios (Fig. 1). The pregnancy continued without further complications until week 31, when the woman contacted the maternity unit by telephone because of bloody discharge and contractions. She could feel the fetus moving as usual and was in good general condition, and was advised to wait and see how events progressed.
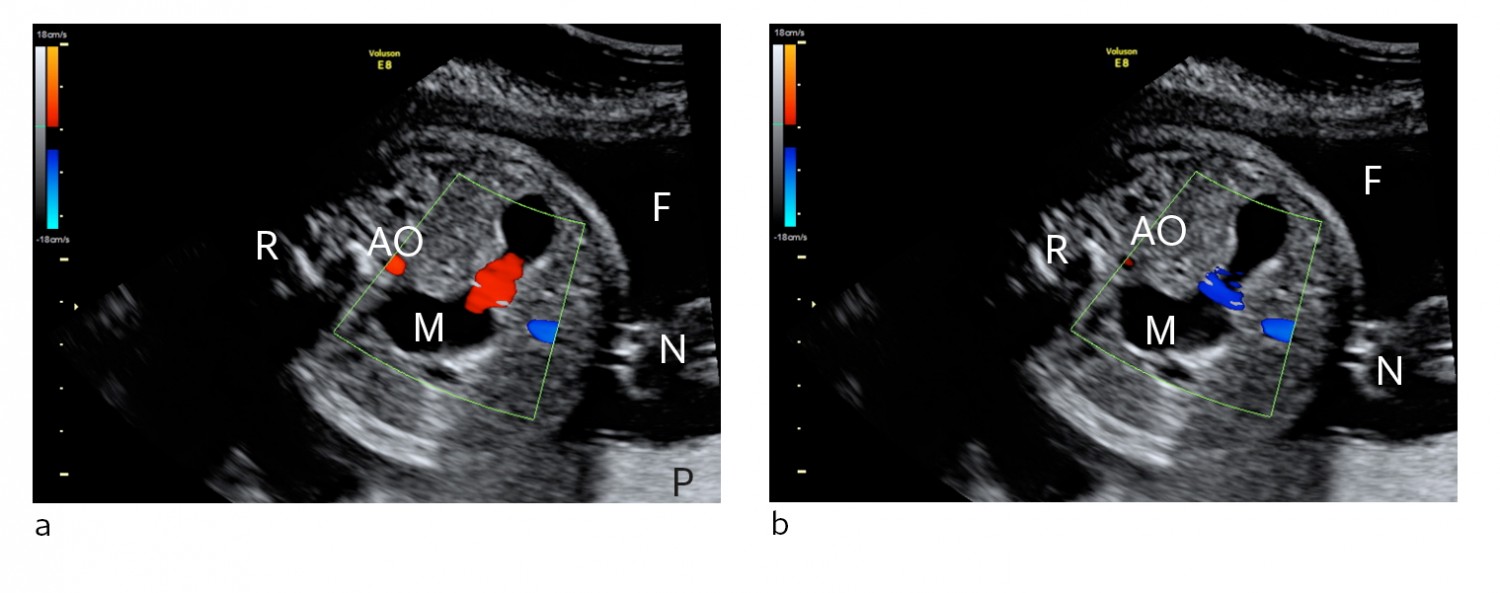
Figure 1 Ultrasound showing cross-section of fetal abdomen at gestational week 29 a) Stomach contents moving towards the probe (red), b) Stomach contents moving away from the probe (blue). A distended stomach (M) with increased motility and movement of gastrointestinal contents back and forth is consistent with distal intestinal obstruction and reflux of contents. M = stomach. R = back, N = umbilical cord, F = amniotic fluid, P = placenta, AO = aorta abdominalis
About a day and a half later, she contacted the maternity unit again as her contractions were coming slightly more regularly. Upon arrival at the unit, her blood pressure was normal (119/76) and her abdomen was soft. She could still feel the fetus moving and was in good general condition. Cardiotocography (CTG) was normal for 20 minutes but the fetal heartbeat then slowed down to about 60 bpm. Technical difficulties were experienced at the same time, and the CTG recording became difficult to interpret (Fig. 2a). An ultrasound examination at 10:18 am initially confirmed bradycardia, but the heart rate quickly normalised. Polyhydramnios was detected.
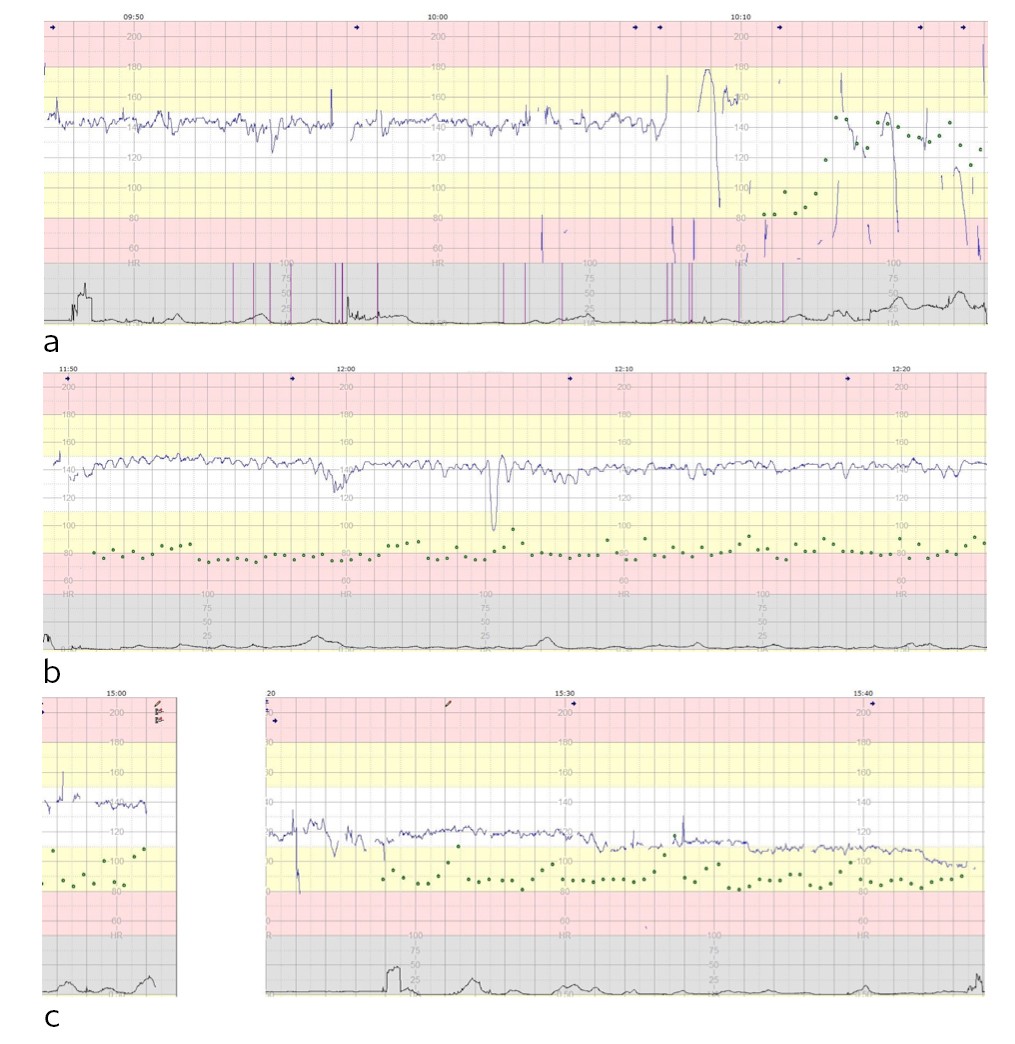
Figure 2 Cardiotocography (CTG) showing fetal heart rate (blue line), contractions (black line), maternal pulse (green dots) and marks indicating fetal movement/kicks (vertical purple lines). A) Normal CTG for 20 min, followed by poor quality recording with bradycardia. B) CTG with sinusoidal pattern indicative of hypovolemia and anaemia. C) CTG with progressive decrease in basal fetal heart rate as a sign of circulatory decompensation
Polyhydramnios may lead to preterm birth. When a pregnant woman is also experiencing vaginal discharge and contractions, it is reasonable to interpret the symptoms as indicating that labour has started. Cardiotocography is a highly sensitive diagnostic test for fetal stress. If CTG is normal, the fetus is not experiencing oxygen deficiency.
The woman was connected to a CTG monitor to measure short-term variability (beat-to-beat variation in fetal heart rate). The basal rate (average fetal heart rate) was 145/min, and accelerations were observed. Short-term variability was normal, at 6.9 ms (normal > 3.5 ms). Maternal symptoms remained stable. The cervix was immature and closed. Betamethasone (glucocorticoid) was administered and pretransfusion samples were taken in case an emergency delivery became necessary. The paediatrician was informed, and the prognosis was discussed for a child with small intestinal atresia born in week 31+5 and requiring surgery only a few days postnatally.
Accelerations are brief increases in fetal heart rate. The presence of accelerations suggests the absence of fetal acidosis and is therefore reassuring. In a scenario with threatened premature labour and known polyhydramnios, drainage of amniotic fluid may reduce the risk of labour commencing, but the procedure itself may also trigger labour (1). Betamethasone treatment may reduce neonatal morbidity and mortality. We therefore opted to postpone amniotic fluid drainage until the betamethasone could be expected to take effect (i.e. after 12 hours).
An ultrasound examination at 11:15 showed that the fetus had normal cardiac function, but was not moving. Measurements of blood flow in the umbilical artery and middle cerebral artery were deemed to be normal. Clinically there was no sign of placental abruption. CTG recording was continued and was perceived to be normal (Fig. 2b).
Umbilical artery Doppler assessment is used in monitoring high risk pregnancies and provides information primarily on the state of the placenta. The technique is used in cases of slow fetal growth, for example, often alongside examination of other fetal or maternal vessels. In the same manner, Doppler assessment of the middle cerebral artery can provide information about fetal condition. High cerebral blood flow velocities would be expected in cases of fetal anaemia due to immunisations, for example.
Short-term variability in the CTG trace decreased from 9.7 ms at 12:57 to 3.0 ms at 14:27. There were no accelerations. This was interpreted as fetal deterioration and led to a further ultrasound examination, which was unchanged from the previous one. CTG was reconnected at 15:20: the basal heart rate had decreased to 120 bpm, but short-term variability had risen to 7.9 ms. The senior consultant on duty was notified. Particular importance was assigned to the improvement in short-term variability. Given the anticipated adverse effects on the child of prematurity combined with intestinal atresia, a decision was made to delay delivering the child. The fetal heart rate had decreased to 100 bpm at 15:42 (Fig. 2c), and after 15:43 it became difficult to detect the heartbeat. In cases of polyhydramnios it can be difficult to detect the heartbeat if the fetus changes position. We decided to move the woman to another room with an ultrasound machine to see whether the fetus had moved and to check the heart rate. Technical problems occurred before the woman was re-examined at 16:03. The examination revealed persistent bradycardia of 60 bpm. Placental abruption was suspected, and the decision was made to perform an emergency caesarean section. The operation began at 16:20.
Preterm cardiotocography can be difficult to interpret. Prolonged absence of accelerations may raise suspicion of pathology, especially when the woman is not in labour. Anaemia and hypovolemia can produce characteristic changes in the heart rate, giving rise to a periodic sinusoidal trace (sinusoidal cardiotocography) (Fig. 2b), but recognising this pattern is easier when such conditions are already suspected. A prolonged decrease in fetal heart rate, despite it being initially within the normal range, indicates circulatory failure in the fetus (Fig. 2c).
The infant, a girl, received an Apgar score of 1/1/1 and had a birth weight of 1 820 g. A large volume of bloody amniotic fluid spilled out of the umbilical cord, and it was not possible to obtain a sample of cord blood for acid-base analysis. It was also unclear whether there were coagula among the fetal membranes.
Bloody amniotic fluid may be a sign of placental abruption or fetal haemorrhage, the former being the more common. Coagula are often visible on the maternal surface of the placenta in cases of placental abruption, but none were seen in this case. The child was pale and the umbilical cord contained no blood, which may indicate fetal haemorrhage.
The paediatrician immediately began cardiopulmonary resuscitation. In the 25 minutes following the birth, 16 ml/kg of sodium chloride at 9 mg/ml and an emergency transfusion of 11 ml/kg blood were administered, along with 0.3 ml intravenous adrenaline at 0.1 mg/ml. The child was intubated and showed stable cardiac function after 24 minutes. Hypothermia therapy was considered but is contraindicated in cases of preterm birth. Upon admission to the neonatal intensive care unit, the child showed limited responsiveness and required respiratory support. The mean arterial blood pressure was 15 mm Hg. Blood samples showed metabolic acidosis with a base deficit of 12.4 mmol/l (normally < 5 mmol/l after caesarean section), lactate 11.1 mmol/l (normally < 4 mmol/l after caesarean section), and haemoglobin was 11.1 g/dl after transfusion.
Dopamine infusion was initiated owing to persistent hypotension. Blood pressure was labile and there was minimal diuresis. A further blood transfusion was given, along with parenteral nutrition with glucose, amino acids and fat. Abdominal X-ray showed air in the stomach and duodenum, but not in the more distal regions, consistent with atresia/stenosis. This finding was not considered relevant to the child’s acute condition. Hb remained persistently low in spite of three blood transfusions, consistent with severe haemorrhage.
Fetal haemorrhage is a rare cause of acute hypovolemia resulting in hypoxic injury. The most common form of fetal haemorrhage is fetomaternal haemorrhage, where the fetus loses blood across the placental barrier into the maternal circulation. Flow cytometry can detect fetal erythrocytes in the mother’s blood. Negative flow cytometry results from a sample taken just prior to the caesarean section allowed us to exclude fetomaternal haemorrhage. Other conditions that may cause fetal hypovolemia are tearing/perforation of fetal vessels in the membranes in association with rupture, or deposition of fetal blood in the placenta (e.g. large cysts or chorangiomas). Haemorrhage in the infant’s internal organs (e.g. liver, stomach/intestines) may also occur.
An EEG performed on the first day of life was almost isoelectric. The results were unchanged when the test was repeated the next day. Diffusion-weighted cerebral MRI showed widespread ischaemic changes in large parts of the cerebrum.
The results indicated very severe brain damage and active treatment was accordingly terminated two days after the birth. The infant died a few minutes after the respirator was disconnected. The relatives were informed about the possibility of an autopsy and gave their consent.
Microscopy of the lungs revealed flakes of amniotic epithelium that had been aspirated in utero (Fig. 3). The stomach was dilated and contained a large volume of material. There was thickening of the pylorus and it was not possible to insert a probe into the duodenum. A spleen sample showed absence of trisomy 13, 18 and 21.
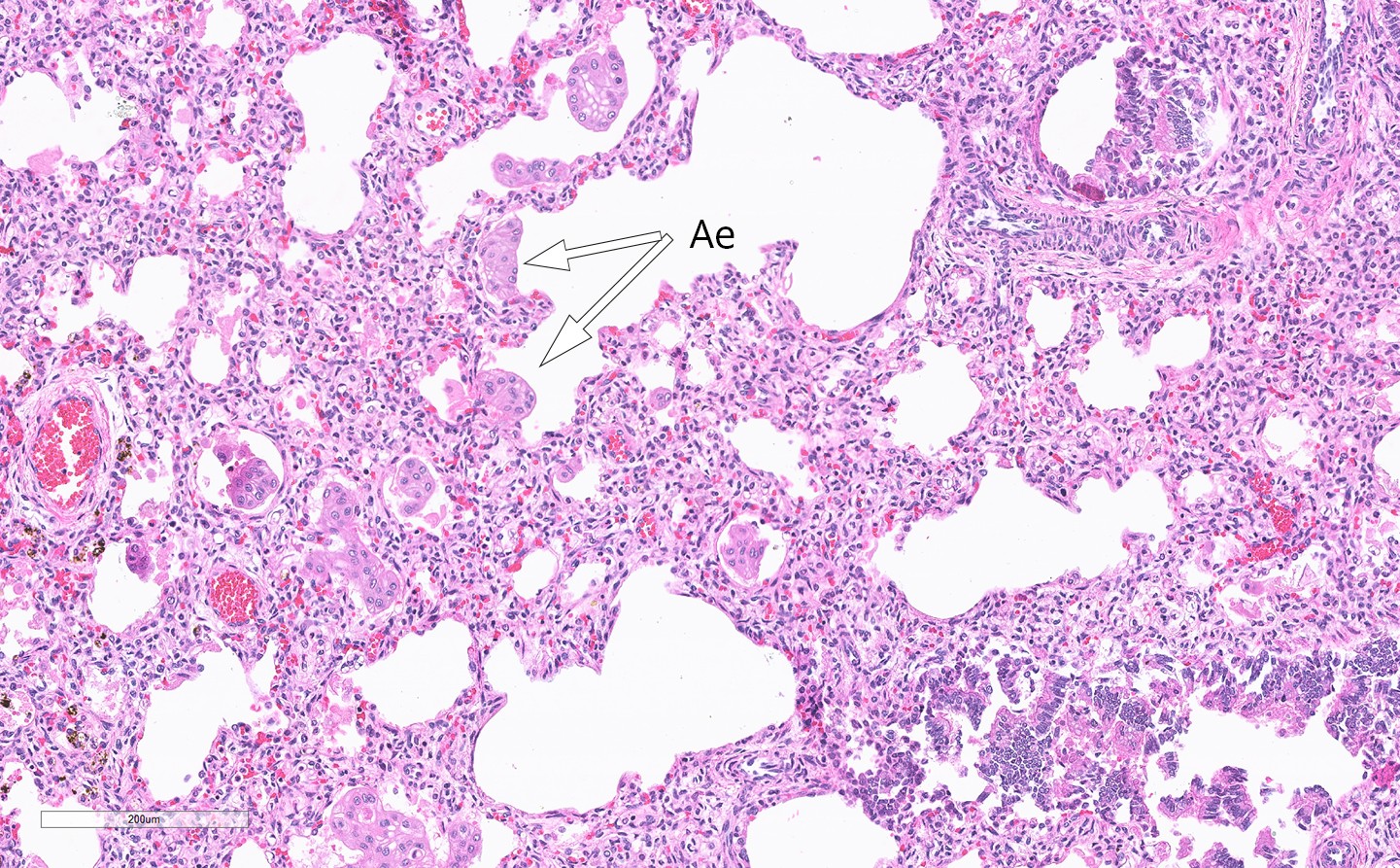
Figure 3 Haematoxylin and eosin-stained lung section with amniotic epithelium (Ae) in the intra-alveolar space
The placenta was intact, with a weight corresponding to the 50th percentile. The maternal (basal) plate was unremarkable with no sign of abruption, and the fetal (chorionic) plate was also normal. The placenta had a typical cross-section, and appeared normal under microscopy. The umbilical cord appeared normal at first, with eccentric insertion, but closer examination revealed that the vessels were exposed and were not protected by Wharton’s jelly, the gelatinous substance that usually protects these vessels (Fig. 4a). Coagulated blood could be seen at the placental end of the cord, but there were no visible tears. Microscopy of the cord revealed two arteries and one vein, but the peripheral arterial walls were very thin and staining for muscle (desmin) revealed destruction of the muscular layer (Fig. 4b). Detached smooth muscle cells were seen scattered around. The amniotic epithelium of the cord had also partially disintegrated. There was no accumulation of nucleated erythrocytes within the fetal blood vessels. Hemosiderin staining was negative. The number of inflammatory cells was not increased, but there were numerous macrophages in the Wharton’s jelly.

Figure 4 a) Cord after formalin fixation. B) Cord cross-section stained with the muscle marker desmin. Destruction of the muscular wall can be seen clearly in two of the blood vessels (arrows)
Examination of the placenta provided no evidence of abruption. The absence of nucleated erythrocytes suggests that the child’s severe anaemia resulted from an acute catastrophic event, a fetal haemorrhage from a damaged umbilical cord artery. Our interpretation of events is that the small intestinal atresia resulted in reflux of stomach contents into the amniotic fluid, which led to necrosis of the cord and Wharton’s jelly, and almost complete destruction of the muscular part of the arteries. This resulted in catastrophic fetal haemorrhage. The presence of aspirated amniotic epithelium in the child’s lungs supports this interpretation (Fig. 3). The cord’s amniotic epithelium had been torn off and then aspirated, explaining its presence in the pulmonary alveolar space.
Discussion
Our patient underwent an emergency preterm caesarean section owing to suspected placental abruption. Subsequent examination of the placenta and umbilical cord revealed the occurrence of a fatal haemorrhage from the latter.
Small intestinal atresia, which is detected in approximately 1 in 5 000 – 10 000 births, is the most common form of congenital intestinal obstruction and may occur with or without other anomalies (2). Small intestinal atresia in combination with other anomalies is associated with more complications and a poorer prognosis. Gestational age at birth also affects the prognosis. Single sporadic cases of isolated small intestinal atresia are most typical, but familial cases have also been reported, both with and without known relatedness. Familial cases consistent with autosomal dominant inheritance have been described, as have families in which there appears to be autosomal recessive inheritance (3, 4). Population studies show high heritability, but no key genetic cause has been identified and multifactorial inheritance is likely (5). The recurrence risk must therefore be assessed in each individual family on the basis of family history. In this family, the recurrence risk will be around 25 %, or somewhat higher if the possibility of autosomal dominant inheritance is taken into account.
Although there is a known association between umbilical cord ulceration and fetal intestinal atresia (6), the literature in the field is limited (6–10). The incidence of umbilical cord ulceration in cases of intestinal atresia is reported to be between 6.5 % (10) and 13.6 % (6). There are several hypotheses about the causal relationship between the two (11). Recent reports propose that gastrointestinal reflux of stomach contents into the amniotic fluid exerts a chemical effect on the amniotic epithelium and Wharton’s jelly, resulting in umbilical cord ulceration (7, 10). It is known that thick meconium may result in cord necrosis (12), which supports the hypothesis that the cause is a chemical process whereby substances in the amniotic fluid destroy the cord. The degree of ulceration does not appear to depend on whether the intestinal obstruction is on the oral or anal side of the major duodenal papilla (7). Haemodynamic changes in connection with birth may increase the risk of ulceration (13).
A Norwegian study showed an increased risk of sudden, unexplained death in fetuses with small intestinal obstruction (14). Bradycardia episodes were recorded prior to fetal death and were interpreted as resulting from vagal overactivity associated with distension of the gastrointestinal tract. It is not clear whether the umbilical cord and placenta were examined in this study.
In our case, fetal bradycardia is thought to have been the result of a reflex response to acute hypovolemia, which is often found in cases of cord rupture (9, 10, 15, 16). In this context, the absence of clinical symptoms such as abdominal pain and vaginal bleeding cannot be considered reassuring (16).
Cord ulceration in association with intestinal atresia is a life-threatening condition with a high risk of mortality or serious sequelae, not least because the fetus has very limited defences against acute hypovolemia (17). Detecting the haemorrhage itself is difficult. The diagnosis should be made when suspected fetal small intestinal atresia is accompanied by abnormal cardiotocography and the absence of maternal symptoms of placental abruption. Doppler ultrasound assessment of the fetal circulation will reveal high blood flow velocity in the middle cerebral artery, suggesting anaemia, but the physiological response of a fetus to severe blood loss (hypovolemia) also depends on factors such as gestational age. The fetal response to severe blood loss over a short period of time has not been fully determined. Rapid delivery with emergency neonatal care and immediate postnatal blood and volume replacement can be life-saving in such cases.
