Fever of unknown origin can be a diagnostic challenge, particularly when symptoms and signs are non-specific and examinations reveal no objective findings. Our patient had previously undergone various surgical procedures. This contributed to making the assessment long and broad-based before a conclusive diagnosis could be reached.
A woman in her thirties was admitted to the Medical Department with suspected infection. For the past nine days she had had a high temperature, 39 – 40oC, reduced general condition, generalised pain in her body, and chills. There were no respiratory or urinary tract symptoms. She had previously had migraine, endometriosis and adenomyosis. She had undergone a laparoscopic hysterectomy and tubal ligation due to endometriosis six months previously. She had subsequently been troubled periodically by pain in connection with her bladder and with urine retention.
She regularly used amitriptyline for tension headache, buprenorphine and paracetamol for endometrial pain, methenamine hippurate as prophylaxis against urinary tract infection and tramadol and ibuprofen as needed. First-degree relatives had suffered malignant melanoma and pancreatic cancer, but otherwise there were no relevant diseases in the family. There was no history of immunological disorders.
Clinical examination yielded normal findings, with the exception of slight tenderness to palpation over the bladder and tenderness to percussion over the right kidney capsule, which she said was usual. The patient was afebrile, and had stable vital signs (blood pressure, pulse, O2 saturation). Urine dipsticks were negative, urine microscopy was normal. Blood tests showed leukocytes 3.2 • 109/l (3.5 – 8.8 • 109/l) and CRP 13 mg/l (< 5 mg/l). The other general blood tests were normal, including sedimentation rate 14 mm/h (< 20 mm/h). X-ray thorax was described as negative, and ultrasound examination of kidneys and urinary tract revealed no pathological findings.
The patient had not been abroad during the past year. Infection was primarily suspected, most likely with focus in the urinary tract. However she had low infection markers and normal urinary findings.
Urine dipsticks and microscopy were repeated and were still negative. The patient was assessed by a gynaecologist, but no new findings were made. The urologist found normal findings with cystoscopy and no residual urine.
Because of the absence of a definite focus of infection while the patient continued to have a high temperature, broader workup was carried out to cover immunological disease, malignancy and infections.
Serological tests for cytomegalovirus, Epstein-Barr virus, parvovirus B19, HIV, syphilis and borrelia, nasopharyngeal PCR with respiratory pathogens in mind, blood cultures, thyroid tests, immunoglobulins, immunological tests and cancer markers CEA (carcinoembryonic antigen) and CA125 (cancer antigen 125) were all negative. General blood tests after two days showed lactate dehydrogenase 214 U/l (103 – 213 U/L) and albumin 33.5 g/l (36 – 48 g/l). The others were normal. CRP and leukocyte level had normalised.
After three days in hospital, there was still no explanation for the patient’s symptoms. She had repeated episodes of high fever, 39 – 40o rectally. Supplementary imaging with ultrasound of the abdomen revealed no pathological findings.
Day 5 following admission there was swelling of the right breast; the breasts had not been examined on admission. Some weeks prior to admission, the woman had had the same symptoms around the right breast and they had resolved spontaneously. Further anamnesis revealed that the patient had had breast implants when she was in her 20s. She later underwent reoperation because of capsule formation around the implants. She had also had Restylane (hyaluronic acid gel) injected into her lips 5 – 7 years previously. Ultrasound of the breast showed loculated anechoic fluid around the right implant.
Could the fluid be related to the fever? The finding was not typical of implant leakage or abscess. A higher echo signal would be expected with implant leakage, and a flaky-looking fluid in the event of an abscess.
Infectious diseases specialist, clinical immunologist and plastic surgeon held an interdisciplinary discussion about the patient. The possibility of a low-virulence microbe, and that the fluid might be reactive, was discussed. In the event of a viral infection, bilateral reactive affection of the implants would be expected.
The plastic surgeon aspirated fluid for diagnosis. Direct microscopy of gram- and acridine-stained specimens yielded negative results, and there was no bacterial growth in the culture. Acridine staining is a fluorescence technique that can be used to detect bacterial DNA. The specimen was not sent for cytological examination. There were still no findings that could explain the fever. The woman was discharged two weeks after symptom onset while awaiting test results.
Three days later she was re-admitted with high fever, 39 – 40oC rectally, reduced general condition and headache. There were no new findings since the previous examination. The exploratory blood tests were still normal.
The patient was again the subject of an interdisciplinary discussion between infection doctors and immunologist. Gastroscopy was recommended to exclude Whipple’s disease, which is a possible differential diagnosis in cases of fever and non-specific abdominal pain.
Echocardiography was ordered with endocarditis in mind, and she was referred for CT of the abdomen and pelvis to exclude complications following earlier lower abdominal operations.
The tests yielded normal findings. The duodenal biopsies revealed no significant pathological findings.
Four weeks after symptom onset, and despite broad workup, there was no evidence of infection, immunological disease or malignancy.
Medication fever was also a possible differential diagnosis, and relevant drugs (amitriptyline and methenamine hippurate) were terminated on a trial basis. After this, somewhat lower temperatures were measured, but the patient still had fever. However she felt a little better. She was again discharged, and early outpatient follow-up was scheduled.
Two days later, the patient reported high fever and pronounced night sweats – she had had to change her bedclothes in the middle of the night.
She had increasing pain in the right breast and tenderness in both axillae, but her infection markers were normal. The right breast was slightly enlarged, but there was no rubor, heat or erythema. She was tender to palpation in the right axilla, but had no palpable lymph nodes.
Because of the patient’s symptoms of night sweats and axillary tenderness, a CT scan of neck and thorax was ordered.
The CT thorax showed enlarged lymph nodes in the mediastinum and lung hila (Fig. 1). Angiotensin-converting enzyme (ACE) was slightly elevated, to 85 U/l (< 60 U/l).
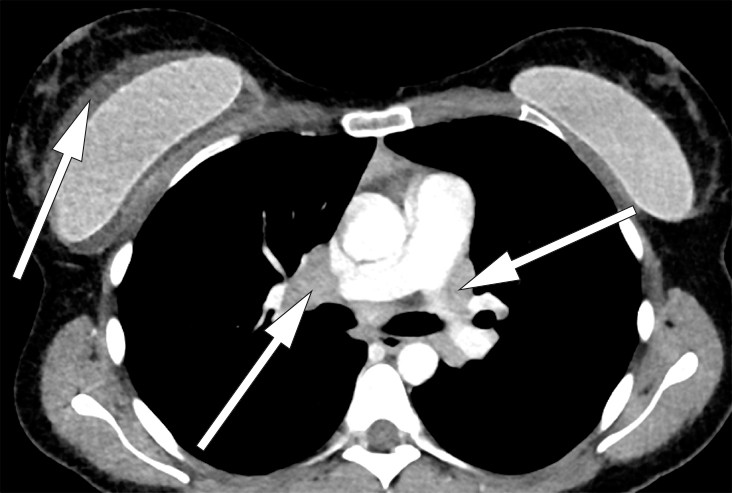
Figure 1 CT thorax with intravenous contrast, sagittal section. A lymph node conglomerate measuring 3 cm is seen in the right hilum. A similar, precarinal lymph node conglomerate was seen (not in the picture). Bilateral breast implants. A fluid rim is seen around the right implant.
Bronchoscopy with endobronchial ultrasound (EBUS) was performed. Fine-needle aspiration cytology (FNAC) from the mediastinal lymph node showed non-necrotising granulomatous inflammation.
Radiologically, the findings could be consistent with sarcoidosis. An elevated ACE level and findings of granulomatous inflammation strengthened this suspicion. However, the clinical findings were not quite typical of sarcoidosis. The most common presentations in cases of lung involvement are cough, dyspnoea and chest pain, often accompanied by lethargy, unwellness, fever and weight loss. Our patient had no respiratory symptoms.
Several case reports have been published about lymphadenopathy induced by silicone implants (1), also in patients who have no symptoms of implant rupture. The most common are local symptoms over the breast implant: cough, lymphadenopathy, reduced general condition and fever. Biopsies of these patients have shown classic granulomatous inflammation and giant cell reaction (1).
We saw granulomatous inflammation in our patient, but no giant cells. There was not sufficient/appropriate material to test the FNAC specimen for silicone in the lymph node.
Since the onset of her illness six weeks earlier, the patient had had fever of 39 – 40oC almost daily. For the past two weeks she had periodically suffered night sweats that made it necessary to change the bedclothes. She had lost 8 kg in six weeks.
Lactate dehydrogenase was normal throughout the course. On the basis of diagnostic imaging, lymphoma appeared unlikely, but in view of the development of pronounced B-symptoms (fever, weight loss and night sweats) a bone marrow biopsy was performed.
The literature also describes rare cases of localised T-cell lymphoma associated with silicone breast implants (2–6).
A bone marrow biopsy revealed normal haematopoiesis. There were no atypical infiltrates, and granulomas were not found. Nor were there any signs of atypia or malignancy.
A CT scan and ultrasound of the breasts were both described as negative with respect to implant rupture. According to the literature, MRI is the preferred modality, as it is more sensitive and specific than ultrasound or CT for detecting rupture (1).
Breast MRI raised suspicion of implant rupture, and there were signs of silicone in axillary lymph nodes bilaterally. It was not possible to assess mediastinal lymph nodes on the breast MRI (Figs. 2, 3).
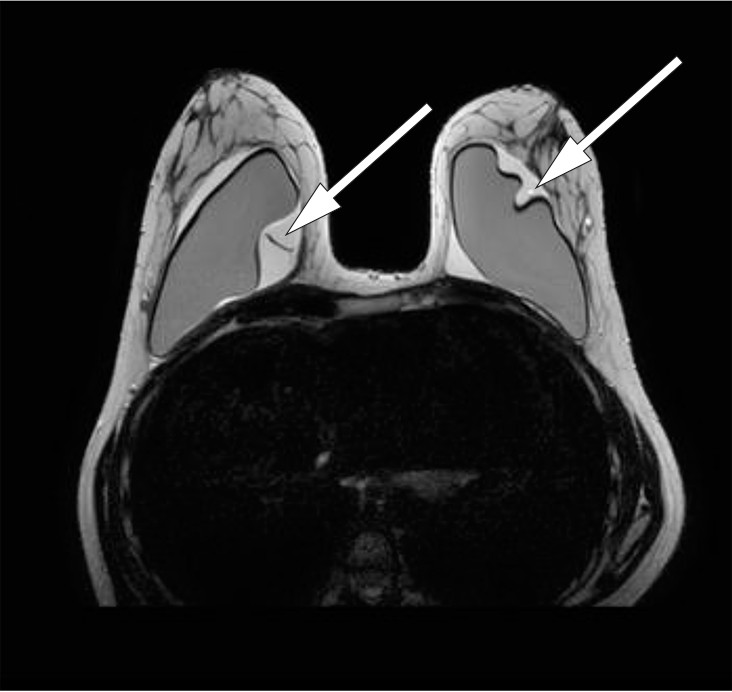
Figure 2 Breast MRI, T2-weighted sequence. Suspected bilateral implant rupture. A substantial amount of fluid surrounding the implants bilaterally
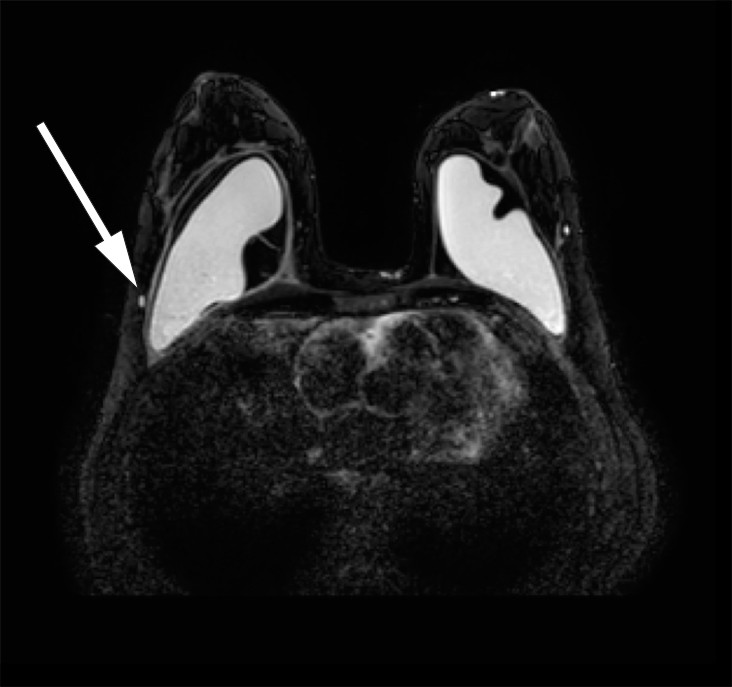
Figure 3 Breast MRI, silicone only sequence. Suspected small quantities of silicone in axillary lymph nodes
At this point, seven weeks after onset, the patient had the implants removed surgically. Both appeared to be intact. Macroscopically, a thickened capsule was seen around the right implant, and there was abundant serous fluid between capsule and implant.
The lung specialist, haematologist and immunologist again held an interdisciplinary discussion about the patient. Her condition could be due to sarcoidosis, an immunological reaction to silicone or lymphoma (large-cell anaplastic lymphoma).
It was decided to wait for the results of histology tests on the implant capsules before carrying out mediastinoscopy in order to secure a histology sample from the mediastinal lymph node, with a view to definitively excluding lymphoma. Prednisolone treatment was deferred until this had been secured. Three induced sputum specimens for tuberculosis testing were negative.
Cytology testing of fluid from the right breast revealed non-specific chronic inflammation. There was no evidence of malignancy. Examination of the operation specimen revealed a thickened capsule on the right side and a somewhat thinner one on the left. There were no signs of implant rupture. A histology sample from the capsule showed granulomatous inflammation without necrosis and hollow “wash-outs” in macrophages, consistent with the presence of silicone (Figs 4 and 5).
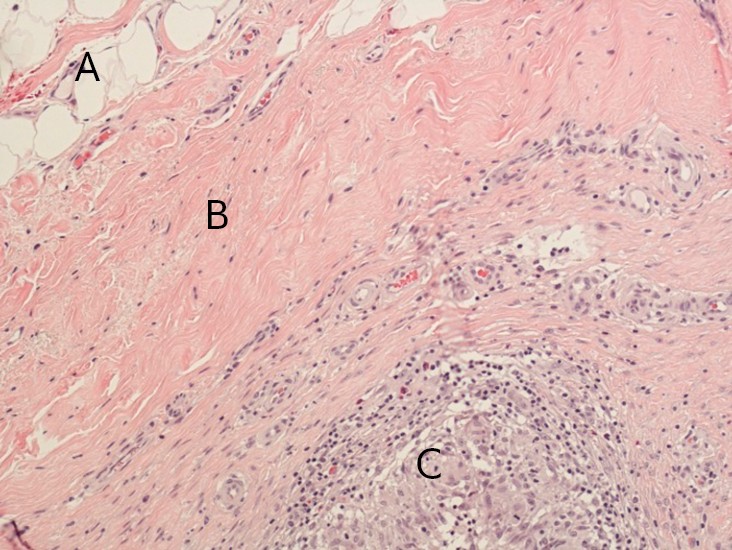
Figure 4 Section through implant capsule, right side: fatty tissue (A), connective tissue (B), granulomas (C). Microscopy shows connective tissue with non-necrotising granulomatous inflammation in all sections from both implant capsules
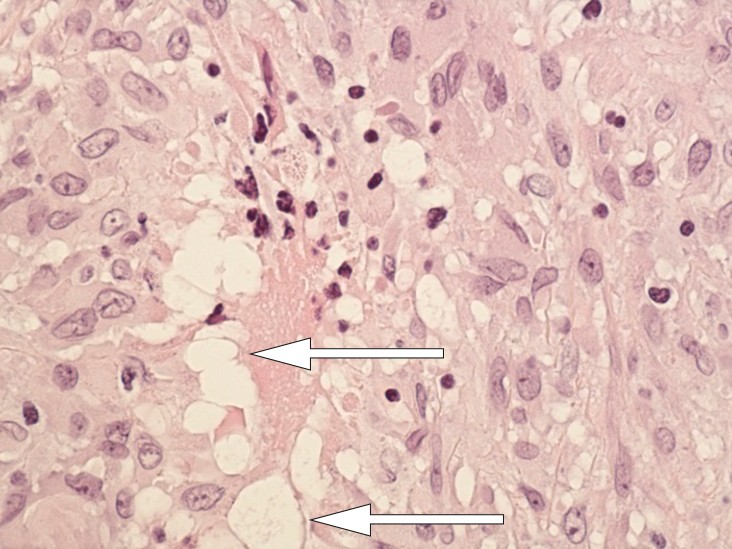
Figure 5 Section through implant capsule: close-up granuloma. There are changes in the macrophages in the form of hollow “wash-outs” consistent with the presence of silicone (arrow)
Prior to mediastinoscopy, nine weeks into the course, the patient developed severe global headache. At the same time she developed peripheral left-sided facial paralysis, and experienced episodes of confusion. Clinical examination indicated involvement of both the left facial nerve peripherally and the trigeminal nerve.
Both lymphoma and sarcoidosis can cause facial paralysis – lymphoma usually as part of more generalised peripheral polyneuropathy, more rarely as isolated facial paralysis (7). The most common neurological finding with sarcoidosis is peripheral facial paralysis. The present deficit might point to sarcoidosis.
MRI of the head showed contrast-enhanced tissue in the trigeminal cavity bilaterally. This was suspected to be a possible manifestation of sarcoidosis. The clinical findings fitted well with the MRI findings, apart from the fact that the patient only had symptoms on the left side. Spinal fluid was negative with respect to virus (herpes simplex virus 1 and 2, varicella zoster, enterovirus and neuroborreliosis), and no bacteria grew in the culture (negative findings with direct microscopy).
Ten weeks had passed since presentation of the first symptom. Sarcoidosis was now regarded as the most probable diagnosis. After interdisciplinary discussion, it was decided that final histological specimens must nonetheless be secured before attempted treatment. Planned mediastinoscopy was therefore performed.
The biopsy from a lymph node in the mediastinum was full of non-necrotising granulomas, consistent with sarcoidosis. There was no sign of lymphoma or silicone (Fig. 6).
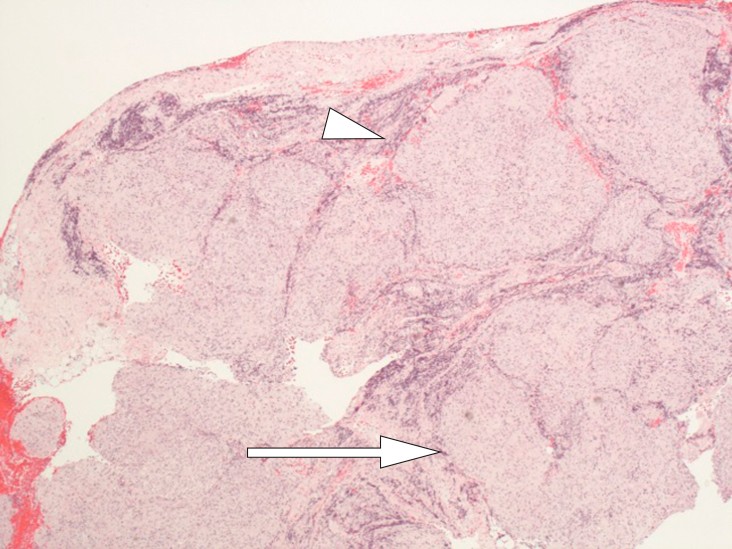
Figure 6 Lymph node with granulomatous inflammation: full of non-necrotising, well-formed epithelioid cell granulomas (arrow), all similar in appearance and maturation with a narrow rim of lymphocytes peripherally as “naked” granulomas (tip of arrow). No evidence of silicon-induced lymphadenopathy. The morphological picture may be consistent with sarcoidosis.
The literature describes spectrometry and electron microscopy as the gold standard for detecting silicone (1). These tests are not available at our laboratory. We concluded that the diagnosis was most probably systemic sarcoidosis.
Eleven weeks after symptom onset, the patient started treatment with prednisolone and Solu-Medrol injections, later start-up of Cellcept.
At follow-up after three weeks she had more energy. She was not experiencing fever or night sweats. Her facial paralysis was abating. Pain relief treatment had been substantially reduced. The patient is receiving further follow-up from the Department of Immunology.
Discussion
Our patient did not present with classic symptoms of sarcoidosis, and several diagnostic debates took place along the way. Because of her short history of illness, infection was initially in focus as the probable cause of her distress. As time passed and more symptoms appeared and the patient grew gradually worse without a diagnosis being reached, broader-based workup was initiated.
A histological picture of non-necrotising granulomas (sarcoid reaction) may occur with sarcoidosis, malignancy and infections. Sarcoidosis is an immunological, multi-system disease that causes a pathological reaction with granuloma formation to an unknown antigen. There are several possible agents.
Despite extensive research, the factor that triggers sarcoidosis remains unknown (8). Usual symptoms are cough, dyspnoea and chest pain, often accompanied by fatigue, weight loss and fever (9, 10). Prevalence is 10 – 20 per 100 000 per year, and young adults are most frequently affected (11).
Granulomatous inflammation can also be seen in connection with migration of silicone from breast implants (1). Silicone may be associated with an immunological reaction called ASIA syndrome (autoimmune/inflammatory syndrome induced by adjuvants) (9). Localised lymphoma in the implant capsule, anaplastic large-cell T-cell lymphoma (ALCL) has been described for clinical manifestations similar to those of our patient (2–6).
A review of the literature reveals several case histories describing lymphadenopathy and granulomatous inflammation in women with breast implants. In 18 cases at the Mayo Clinic, a clinical presentation similar to that of our patient is described – fever, fatigue, focal symptoms over the implant and lymphadenopathy in axillae and hila. Thirty-four per cent of these patients had symptoms of implant rupture; the remainder were asymptomatic (1).
Silicone that migrates outside the breast initiates inflammation and granuloma formation (12). Migration to axillary lymph nodes is most common. Lymph drainage from the breast occurs through three main routes: axillary, transpectoral and internal mammary lymphatic pathways (13). Silicone migration may follow these paths, but it may also spread retrogradally or along other pathways (14). Rare cases of pneumonitis have also been observed (15–17).
Shoenfeld and Agmon-Levin describe the new ASIA syndrome, which is triggered by foreign bodies. It is assumed, for example, that silicone may induce immunological disease (18). The existence of the syndrome is a matter of contention (19, 20).
We find several case reports describing granulomatous inflammation and cutaneous sarcoidosis after injection of silicone for cosmetic purposes (21–25). Remission of assumed sarcoidosis after removal of silicone breast implants has also been described (26). Silicone has been shown to induce autoimmunity in various animal models (27), but no definite connection has been found between autoimmunity and silicone implants in humans (19, 20, 28, 29).
Detection of silicone in tissue or granulomas does not exclude a diagnosis of sarcoidosis (25).
We cannot conclude that the silicone implants affected our patient. She had granulomatous inflammation in connection with the implant – hollow “wash-outs” as signs of silicone leakage, MRI findings of assumed silicone in axillary lymph nodes and focal symptoms that may indicate a connection. What points towards systemic sarcoidosis is the involvement of the central nervous system, and the fact that no sign was found of giant cells or silicone in the mediastinal glands.
Our conclusion is systemic sarcoidosis, but the part played by the silicone implants in the disease picture remains uncertain.
