Choosing appropriate medication doses for pregnant women is a difficult balancing act, as the mother’s need for treatment must be weighed against the risk of fetal harm. The latter is frequently considered to be the most pressing concern, with the result that drugs are discontinued or doses reduced. It is perhaps less well known that pregnant women often need higher medication doses than those who are not pregnant.
Many women take medications while pregnant, either because of conditions related to the pregnancy or due to other illnesses (1, 2). In Norway, almost 80 % of pregnant women report having used one or more medications during pregnancy (3). Reference books such as the Norwegian Pharmaceutical Product Compendium and the RELIS database (4) can provide information about the safety of taking medication during pregnancy and breastfeeding. However, such recommendations are often inconclusive due to lack of data (5).
We know very little about how a pregnant woman’s body deals with specific drugs. Absorption, distribution, metabolism and excretion of drugs may be affected by the physiological changes that occur during pregnancy. These factors may in turn impact on the drug dose needed by the pregnant woman (2). If dose recommendations are extrapolated from non-pregnant to pregnant women without taking account of the physiologic changes of pregnancy, there is a risk of underdosing or overdosing. It is our impression that this is a little-known issue among clinicians, and that it is often overshadowed by discussions surrounding drug teratogenicity.
This article provides an overview of the impact that physiological changes in pregnancy have on drug pharmacokinetics, and presents some examples of medications that may require dose adjustment during pregnancy. We also give some general advice on how to plan for and monitor drug doses in pregnant women. The article is based on literature searches in PubMed in addition to the authors’ own experience and research in this field.
Pharmacokinetics in pregnant women
Most organ systems are subject to physiological changes during pregnancy, and many of these may affect the pharmacokinetics of drugs. Reduced gastrointestinal motility and increased gastric pH may affect the absorption of drugs. Declining concentrations of drug binding proteins may, in combination with an increase in total body water and plasma volume, lead to higher distribution volumes and reduced serum concentrations of some drugs. The concentrations of albumin, which binds drugs that are weak acids, and of α1-acid glycoprotein, which binds drugs that are weak bases, decline. Changed activity of various hepatic drug-metabolising enzymes may result in reduced, unchanged or increased concentrations of drugs. The activity of both cytochrome P-450 (CYP)-enzymes and glucuronidation enzymes will be affected. Increased renal blood flow and a higher glomerular filtration rate will increase the excretion of drugs relying mainly on renal elimination. An overview of the main pharmacokinetic changes that occur during pregnancy is given in Figure 1 (2, 6, 7, 8). The changes will normally increase gradually throughout pregnancy, reach their maximum level in the second or third trimester and normalise within 1–2 weeks of delivery (2, 9).
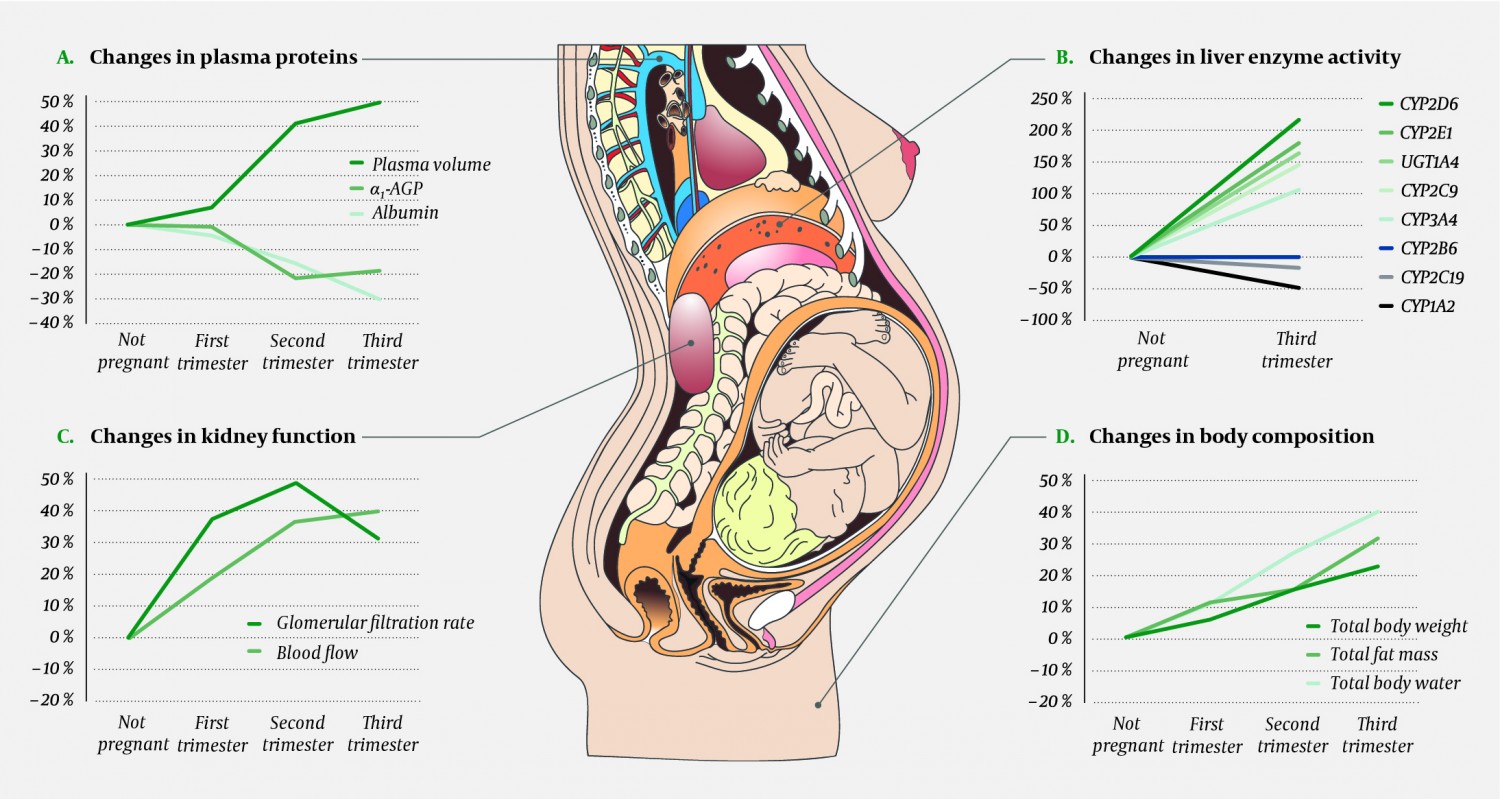
Figure 1 Some key physiological changes in pregnant women that affect the absorption, distribution, metabolism and excretion of drugs. Panel A shows how pregnancy leads to increased plasma volume and a similar fall in the concentration of drug-binding plasma proteins. Panel B shows activity changes for important drug-metabolising enzymes. Cardiac output increases during pregnancy, which in turn leads to increased renal (panel C), hepatic and pulmonary blood flow. Panel C also shows the changes in glomerular filtration rate. Panel D shows pregnancy-dependent changes in body composition that may affect the volume of distribution for drugs. Data adjusted from references (2, 6, 7, 8). α1-AGP = α1-acid glycoprotein (orosomucoid), CYP = cytochrome P-450, UGT = uridine diphosphate glucuronosyltransferase. Illustration: p6 m5/stock.adobe.com.
Based on knowledge about the pharmacological properties of individual drugs and the physiological changes presented in Figure 1, one may attempt to estimate how pregnancy will affect maternal drug concentrations. As a rule of thumb, the drug’s elimination route is the most crucial factor to consider. If the drug is mainly excreted unchanged through the kidneys, serum concentrations will often decline by almost 50 % during the pregnancy (Fig. 1, panel C) (6). If the drug is mainly eliminated by the liver, a concentration fall of the same magnitude (or greater) may occur, but the concentration may also remain unchanged, or in some cases increase (6). This depends on which enzyme or enzymes are most important for the metabolism of that particular drug, because changes in enzyme activity vary in magnitude and direction during pregnancy (Fig. 1, panel B) (6). Increased hepatic blood flow will also play a role in the degradation rate of some drugs.
Multiple physiological changes often affect a single drug during pregnancy, for instance with one drug-metabolising enzyme being inhibited while another is induced (this is the case for the antipsychotic drug olanzapine (10)), or with an increase in both hepatic and renal elimination (this is the case for the antiepileptic drug lamotrigine (11)). The net effect of these changes can be difficult to predict, and there can be considerable individual variation. Consequently, in order to provide the best possible dose recommendations for pregnant women, theoretical reflections concerning the drug’s pharmacokinetic properties will not suffice. We need dedicated pharmacokinetic studies in pregnant women, and preferably serum concentration measurements from the patient in question. The clinical effect must also be closely monitored.
What do these changes mean for pregnant women?
The examples given in Figure 1 and Table 1 (2, 9, 10, 11, 12, 13, 14, 15, 16) show that drug concentrations usually decline during pregnancy, most often due to increased hepatic metabolism or increased renal excretion. These changes may cause the patient’s “usual” pre-pregnancy dose to become too low during pregnancy. For some drugs, such as lamotrigine (11), it has been clearly shown that declining concentrations in pregnancy may cause therapeutic failure. However, for most other drugs this relationship has been less well researched (2).
Table 1
Examples of pharmacokinetic changes during pregnancy and their practical consequences. The drugs listed in the table are only intended as examples and do not reflect preferred treatment options. The practical advice is based on an individual risk-benefit-assessment that suggests the pregnant woman should take the medication and maintain a stable serum concentration. CYP = cytochrome P-450, UGT = uridine diphosphate glucuronosyltransferase.
|
Medication (reference)
|
Indication
|
Pharmacokinetic change during pregnancy
|
Practical consequences
|
|
Metoprolol (2, 9)
|
Hypertension, migraine etc.
|
Liver metabolism via CYP2D6 increases by up to 4 times or more, thereby lowering the serum drug concentration.
|
Treatment for hypertension should involve blood pressure monitoring. If appropriate, the serum concentration of the drug should also be measured.
|
|
Methadone (9, 12)
|
Opioid dependency
|
Liver metabolism via CYP3A4 increases, thereby lowering the serum drug concentration.
|
The dose requirement may increase. The clinical effect should be monitored. If appropriate, the serum concentration of the drug should be measured.
|
|
Cefazolin (2, 6)
|
Infections
|
Increased distribution volume and increased renal excretion lower the serum drug concentration.
|
The dose should be increased to ensure appropriate concentration at the infection site.
|
|
Indinavir (2, 13)
|
HIV/AIDS
|
Liver metabolism via CYP3A4 increases, thereby lowering the serum drug concentration.
|
The virus level should be quantified. If appropriate, the serum concentration of the drug should be measured.
|
|
Lamotrigine (2, 11)
|
Epilepsy and bipolar disorder
|
Increased glucuronidation via UGT1A4and increased renal excretion lower the serum drug concentration.
|
The serum concentration should be measured, preferably monthly, and the dose increased if required.
|
|
Lithium (14)
|
Bipolar disorder
|
Increased renal excretion lowers the serum drug concentration.
|
The dose requirement may increase. The clinical effect should be monitored and the serum concentration measured, for example monthly.
|
|
Escitalopram (15)
|
Depression etc.
|
Little or no change in liver metabolism.
|
Dose adjustment is not normally needed.
|
|
Quetiapine (10)
|
Schizophrenia and bipolar disease
|
Liver metabolism via CYP3A4 increases, thereby lowering the serum drug concentration.
|
The dose requirement may increase. The clinical effect should be monitored and the serum concentration measured, for example monthly.
|
|
Caffeine (16)1
|
-
|
Reduced liver metabolism via CYP1A2 increases the serum drug concentration.
|
The drinking of coffee may feel unpleasant because pregnant women achieve higher caffeine concentrations than others. Coffee consumption should be limited during pregnancy.
|
The recommendation is often that pregnant women should be treated with ‘the lowest effective dose’ of any drug. The problem with this recommendation is that it risks reducing the dose at a time when the dose requirement actually increases (Fig. 2). If a decision has been made that a pregnant woman should use a particular medication, it is important to emphasise that the dose must be effective, not only low. Otherwise there is a risk that the child is doubly exposed, both to the drug and to the harmful effects of the mother’s disease (for example, both to an antiepileptic drug and to maternal epileptic seizures).
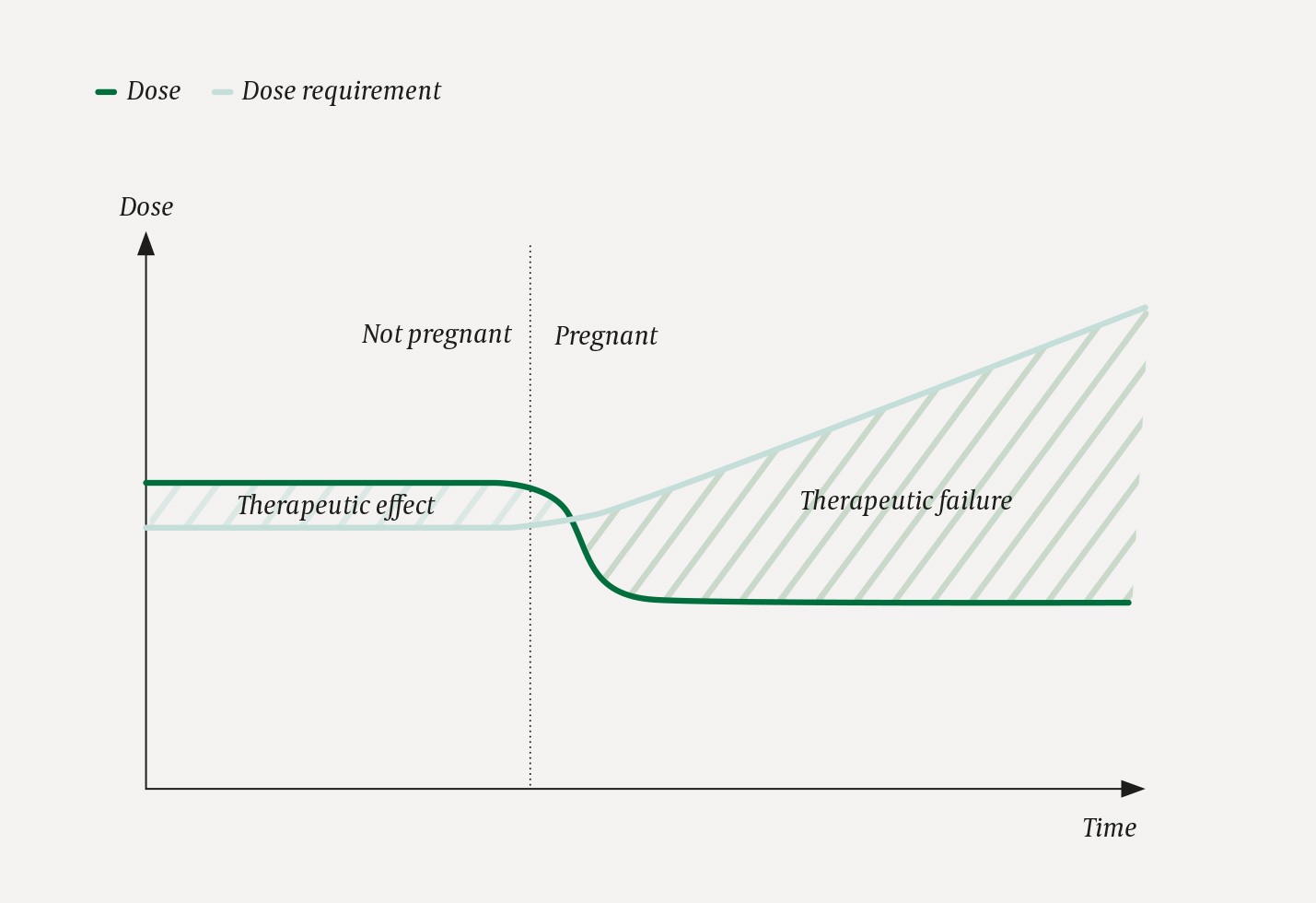
Figure 2 Risk of therapeutic failure in pregnant women. The figure shows why pregnant women are at risk of suboptimal dosing and therapeutic failure. In this hypothetical scenario the patient takes a drug X. The dose is indicated by the green line. When the patient discovers that she is pregnant, she agrees with her doctor to reduce the dose of X, out of concern for the fetus. At the same time, the woman’s dose requirement increases due to increased elimination of X. Both events (reduced dose and increased dose requirement) contribute to widening the gap between the dose she needs and the dose she receives, thus putting her at risk of therapeutic failure.
Practical advice
In general, little is known about pharmacokinetic changes and dose requirements during pregnancy, yet there are some good review articles on the topic (2, 6, 9). For a busy clinician, however, the simplest and safest way to proceed will be to seek advice from the regional medication information centres (4) or from a department of clinical pharmacology, in order to obtain up-to-date information regarding the safety of the child and the mother’s likely dose requirement. As explained above, it is possible to estimate the mother’s serum concentrations during the pregnancy based on knowledge about the drugs’ elimination route (and any pregnancy studies that might exist), and thereby plan for dose adjustments. In addition to regular clinical assessments during the course of pregnancy and measurements of any effectiveness markers (such as blood pressure, INR etc.), therapeutic drug monitoring may be a useful tool as the pregnancy progresses. For any drug that can be measured (see the Norwegian Pharmacology Portal (17) for a national listing), we recommend that a measurement is taken before or as early as possible during pregnancy to provide a reference point for later comparison (a so-called baseline test). Further measurements can then be made monthly, every second month or every trimester, depending on the drug, the indication and the practical circumstances. If a dose increase is needed, it is important that the pregnant woman is kept well informed, because many find that it goes against reason for doses to be increased rather than decreased during pregnancy. And if the dose is increased during pregnancy, it must be reduced immediately after delivery to avoid overdosing (11). Finally, it is important to remember that irregular drug intake, self-imposed dose reductions and discontinuation of drug regimens regularly occur in pregnant women (18). In case of low serum concentration or uncertain effect, adherence should be investigated before the dose is increased.
