When cancer patients develop new neurological symptoms, it is important to think broadly in terms of differential diagnostics. The cancer itself, but also the treatment and circumstances surrounding a serious diagnosis, may all affect the patient and make determining the cause of the health changes a challenge. Supplementary information about clinical findings and relevant treatment by cooperating specialists may be crucial for arriving at the correct diagnosis.
Posterior reversible encephalopathy syndrome (PRES) is a rare clinicoradiographic disorder characterized by acute neurological symptoms with typical neuroimaging findings of vasogenic oedema in posterior regions of the brain. This complication is linked to a number of medical conditions, and is increasingly being documented as a side effect associated with a number of therapeutic agents. We present a case of PRES as a result of treatment with the vascular endothelial growth factor (VEGF) multikinase inhibitor, regorafenib.
A man in his 50s with known metastatic colorectal cancer presented with headache, vomiting, altered mental state, reduced hand coordination and dexterity, and a homonymous inferior quadrantanopia. Symptoms developed soon after completion of the third regorafenib therapy cycle. Cerebral MRI demonstrated signs indicative of PRES with bilateral vasogenic oedema in the occipitotemporal regions. Regorafenib was subsequently discontinued and the patient’s condition improved gradually, with normalisation of his neurological symptoms within a month. PRES has been linked to VEGF treatments, particularly sorafenib, sunitinib and pazopanib, albeit rarely. However this is the second reported case linking regorafenib with PRES. PRES is usually associated with a good prognosis. However, delayed diagnosis and treatment may lead to permanent neurological symptoms, higher morbidity and in rare cases mortality. Therefore increased awareness of this condition is vital.
A previously healthy man in his 50s, was found to have rectal cancer with metastasis to the liver. After a multidisciplinary assessment he started neoadjuvant chemotherapy in the form of fluorouracil (5-FU), oxaliplatin and calcium folinate (FLOX) prior to radiotherapy, with a favourable radiological effect on liver metastases. The patient then underwent an uncomplicated operation with rectal resection and end colostomy to prevent complications from the primary tumour. After the operation, he continued on the same chemotherapy, but quickly developed bone marrow toxicity with thrombocytopenia of 84 · 109/l (reference range: 130–400) and neutropenia of 1.0 · 109/l (1.7–8.0) despite reduced doses of cytostatics and tapering of oxaliplatin.
After six months of cytostatic therapy, including long breaks between courses due to thrombocytopenia, the cancer progressed, with further spreading to the lungs. His chemotherapy was changed to fluorouracil, irinotecan and bevacizumab. Bone marrow toxicity in the form of thrombocytopenia recurred after only one course. Treatment was therefore changed to monotherapy with irinotecan and cetuximab, an epidermal growth factor receptor inhibitor. The conclusion of the first evaluation was progression of the patient’s cancer. Radiology demonstrated progression of liver metastases and blood tests showed an increase in carcinoembryonic antigen (CEA) level from 42 μg/l to 71 μg/ l (< 5) and increases in liver test values with alanine aminotransferase (ALT) 76 U/l (< 70), alkaline phosphatase (ALP) 300 U/l (< 105), gamma glutamyl transferase (GT) 860 U/l (< 115), lactate dehydrogenase (LD) 400 U/l (< 205) and aspartate aminotransferase (AST) 93 U/l (< 45). This indicated therapeutic failure and poor tolerance, even after third-line therapy. Therefore, following a break of one month, oral treatment was started with regorafenib, a multikinase inhibitor, with a daily three-week course followed by a week’s pause.
On evaluation after completion of two cycles (eight weeks in all) the patient had declining levels of the cancer marker CEA (from 92 μg/l at the start to 32 μg/l (< 5)). The radiological workup showed a distinct liver response with regression of metastases with newly developed necrosis. Blood tests showed improvement of some of the elevated liver values (ALT 80 U/l, ALP 378 U/l, GT 873 U/l, LD 284 U/l and AST 87 U/l). There were no signs of bone marrow toxicity, which had previously developed rapidly during treatment. During the courses, he had complained of mouth sores, dry skin and pain in the palms of his hands and soles of his feet.
After completing the third cycle, a total of 12 weeks of treatment, the patient came to the cancer clinic for a scheduled check-up. He reported increasing headache since the beginning of the third cycle, and difficulty in carrying out daily activities. During the course he had gradually developed pronounced blisters on the soles of his feet and palms of his hands, but these were now in remission. According to his wife, he was more irritable, and she also noticed that he apparently did not see food on the lower part of the plate, eating only what was on the upper half. He expressed increasing fear of being alone and had sudden episodes of crying. He reported exacerbation of these symptoms during the past week, with a worsening, very intense and persistent headache accompanied by vomiting later in the day. A head CT was ordered on suspicion of brain metastases. The patient was also referred to an ophthalmologist and the regorafenib course was temporarily stopped.
Brain metastases in cases of widespread cancer may give rise to symptoms such as headache, usually morning headache, with accompanying nausea and vomiting. This may be due to elevated intracranial pressure, owing to the mass effect of the tumour, oedema around the tumour, and/or secondary development of hydrocephalus. Depending on the location of the tumour, focal neurological symptoms such as palsy, visual field impairment and apraxia are common. Brain metastases may also cause focal and/or secondary generalised epileptic seizures, sometimes as a presenting symptom. There is often also non-specific cerebral dysfunction with symptoms such as fatigue, irritability, personality changes or psychiatric symptoms. Metastases in the posterior cranial fossa often cause double vision, vertigo and coordination impairment (1, 2).
If there is haemorrhaging in the tumour, symptoms can develop acutely, mimicking a stroke (1). Brain metastases are more common than primary intracranial tumours, and are seen most frequently with primary lung cancer, followed by breast cancer, malignant melanoma, gastric cancer, genitourinary cancers, and less frequently with colon and rectal cancer (2). A brain MRI scan is the most sensitive means of depicting brain metastases. As a rule they appear as round, well-defined tumours with variable signal, depending on the type of primary tumour and presence of necrotic tissue. Metastases usually show contrast enhancement, and may be surrounded by pronounced oedema (1).
Head CT scans of the patient were taken with and without intravenous contrast. Low attenuation indicative of oedema in the white matter was seen in the occipitotemporal regions, being more pronounced on the right side with a mass effect and displacement of the midline towards the left (Fig. 1).
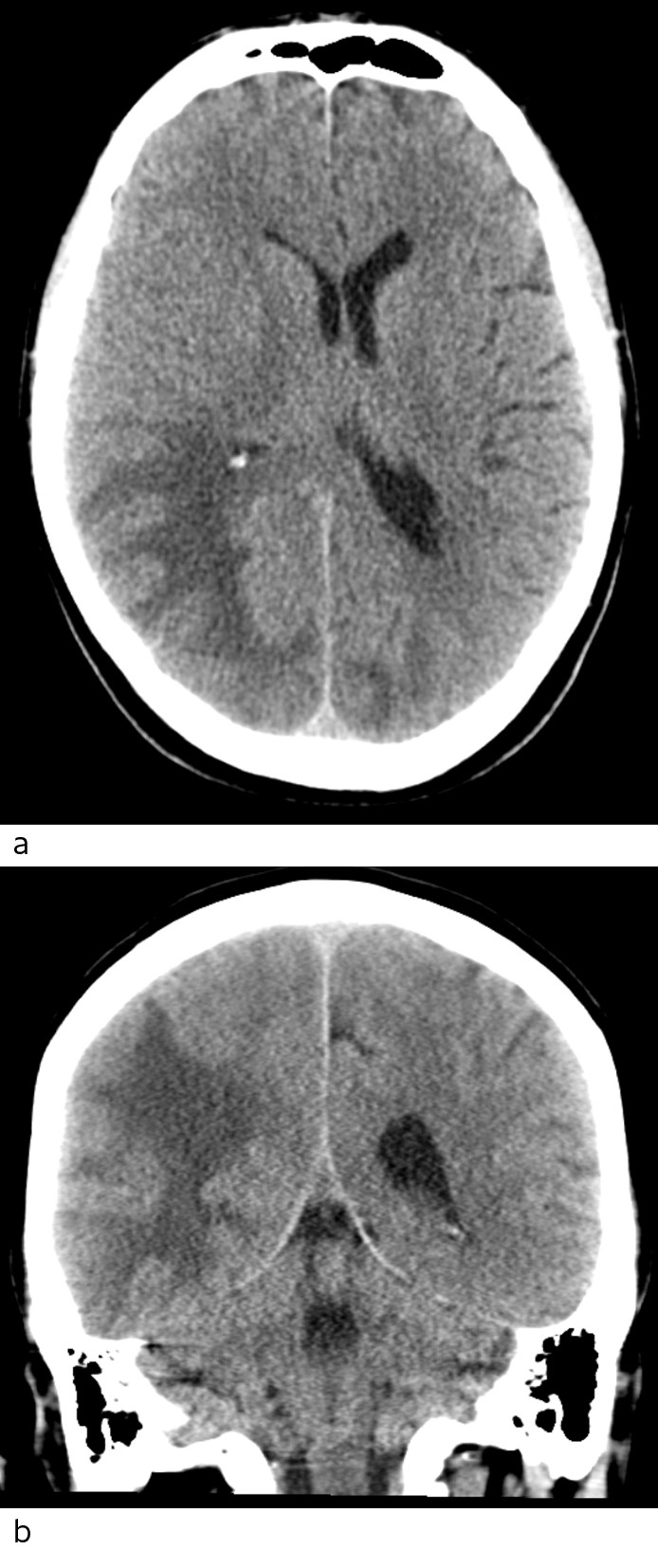
Figure 1 Head CT scan axial section (a) and coronal section (b) show extensive low-density changes that may be consistent with oedema occipitotemporally on the right side and occipitally on the left side. No haemorrhaging is seen.
The patient was admitted to the Department of Neurology. A clinical examination found him conscious, lucid and orientated with respect to time, place and person. He had blood pressure of 132/88 mm Hg and a regular pulse of 70 beats/min. He was afebrile and had a normal respiratory rate. Using Donder’s method, the examining doctor found a restricted field of vision over the left, lower quadrant. The patient reported numbness and paresthesia in the soles of his feet and that his feet felt cold. There was reduced sensibility to touch and pinpricks from knee level and distally, a consequence of chemotherapy-induced peripheral polyneuropathy (a known sequela of prior chemotherapy). He had normal muscle strength in his extremities, but reduced coordination and dexterity in both hands, with a slight dysdiadochokinesia in the left upper extremity. His reflexes were normal, with a bilateral downward plantar response. Romberg’s test was pathological. Otherwise normal neurological and somatic examinations. Blood tests showed thrombocytopenia 106 · 109/l (130–400), sedimentation rate 28 mm/t (< 20), CRP 39 mg/l (< 5), ALP 353 U/l (< 105), GT 661 U/l (< 115), LD 269 U/l (< 205) and CEA 49 μg/l (< 5). Blood tests were otherwise normal, including differential leukocyte count.
An ophthalmological examination showed a left homonymous inferior quadrantanopsia; otherwise normal findings. In light of symptoms of increased intracranial pressure with brain oedema detected on the CT scan, brain metastases were suspected. Treatment with high-dose per os corticosteroids was therefore started and the patient was referred for a cerebral MRI.
The day after admission, the patient was already showing signs of clinical improvement and was subsequently discharged pending the MRI results. The patient’s cognitive function was assessed by an occupational therapist using the Trandex-score, with the patient scoring 46/60 points; points were deducted for orientation, memory, concentration and abstract thinking. This indicated a slight cognitive deficit.
The MRI with intravenous contrast (Fig. 2) showed increased signal in the white matter in the right temporal and occipital regions, and in the medial left occipital region on T2 weighted and FLAIR sequences. There was no restriction on a diffusion-weighted series (as usually seen in stroke, abscess and tumours), but an increased apparent diffusion coefficient (ADC), consistent with vasogenic oedema. There was no pathological contrast enhancement or contrast-enhanced lesions, which would point to metastases, abscess or primary brain tumour, but numerous punctate haemorrhages were seen in the cortex of the corresponding areas on susceptibility-weighted sequence. MRI spectroscopy showed normal metabolite distribution, which also weighed against tumour.
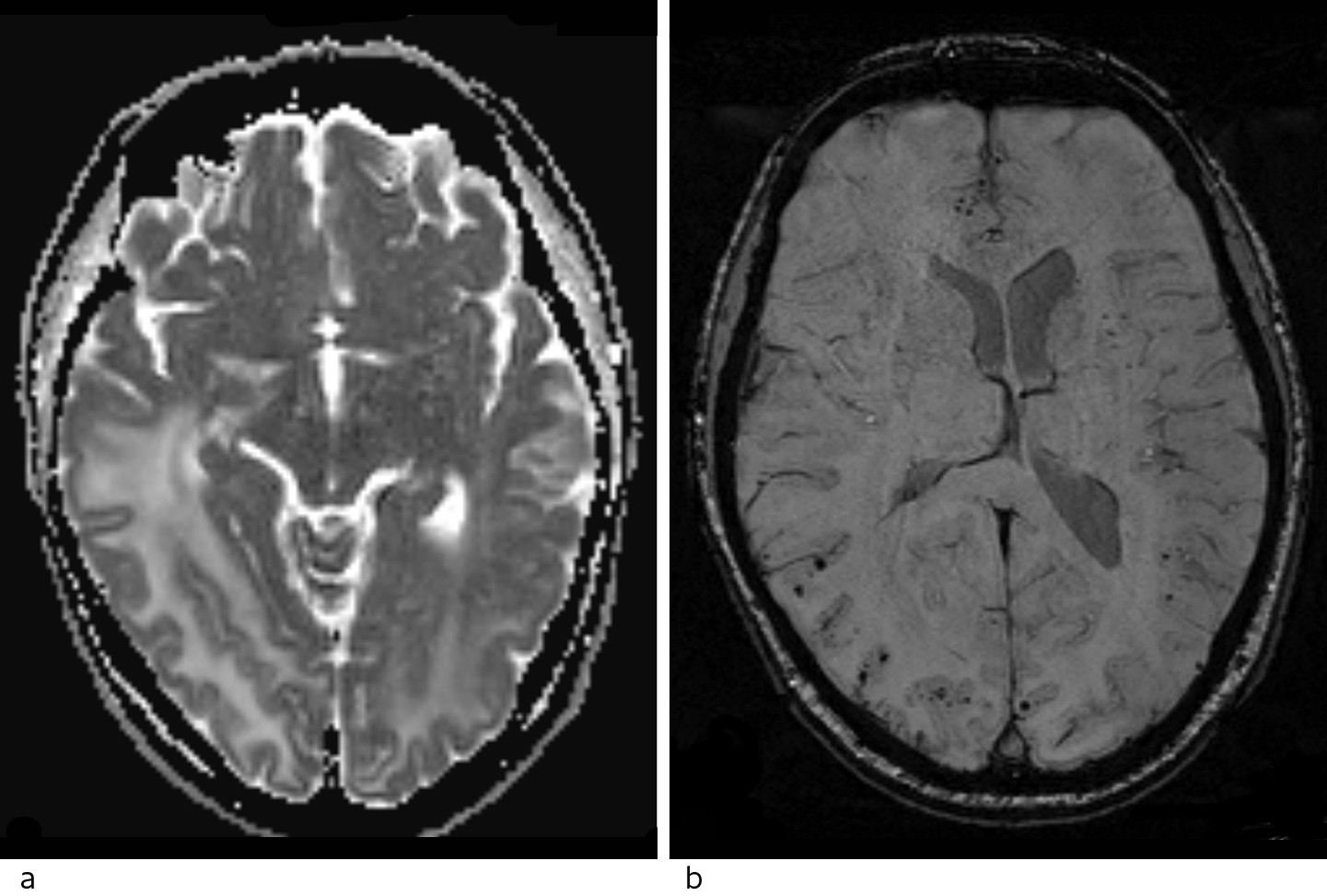
Figure 2 Head MRI. a) ADC map with high values consistent with vasogenic oedema. b) Susceptibility-weighted imaging (SWI) shows numerous punctate lesions with a very low signal, consistent with microhaemorrhages spread through the cortex of both hemispheres, predominantly in the right occipital lobe.
In terms of differential diagnostics this could be consistent with gliomatosis cerebri, progressive multifocal leukoencephalopathy (PML) or posterior reversible encephalopathy syndrome (PRES). Gliomatosis cerebri is an extremely rare brain tumour originating from astrocytes. It typically grows diffusely in the white matter, affecting at least three lobes. This tumour usually presents with a benign clinical picture, but an extensive radiological lesion load (3).
Progressive multifocal leukoencephalopathy is a demyelinising brain disease caused by reactivation of latent John Cunningham virus (JCV, a human polyoma virus) in patients with a compromised immune system (4). The condition can cause subacute neurological symptoms such as cognitive impairment, motor symptoms such as hemi- or monoparesis or ataxia, or visual disturbances such as hemianopsia and diplopia (4). The immune system of our patient may have been suppressed by the regorafenib therapy, and by earlier chemotherapy, but he had no leukopenia on admission, nor was immune suppression found at check-ups during his treatment with regorafenib.
The polymerase chain reaction test was negative for John Cunningham virus. This made progressive multifocal leukoencephalopathy less likely.
In light of his medical history, the course of the disease and MRI findings of vasogenic oedema and multiple small cortical haemorrhages in the occipital regions, we concluded that the diagnosis of PRES was the most probable, caused by regorafenib therapy for his cancer.
Regorafenib and corticosteroid therapy were discontinued, and the patient experienced spontaneous, swift improvement with headache regression, normalisation of his field of vision, dexterity and balance, without any other interventions. An outpatient check-up a month later found full restitution of his neurological symptoms and normal clinical status. MRI showed considerable regression of the lesions. A head CT scan conducted three months later still showed modest residual changes around the posterior horn of the right lateral ventricle and regression of the midline displacement. The patient complained of more memory difficulties, and there were clinical, biochemical and radiological signs of progression of his cancer. He died of cancer three months later.
Discussion
PRES is usually considered a reversible clinicoradiographic disorder. The incidence is not known, but the condition is considered to be underdiagnosed. It affects all age groups, from 2 to 90 years, and appears to be more widespread among women because of its association with preeclampsia, although this has not been published (5). PRES has previously been described in Tidsskriftet in a child with convulsions and sudden loss of vision during a course of chemotherapy (6). Clinically, the condition may present with a variety of neurological manifestations, normally with a (sub)acute onset that extends from hours to weeks. Presenting symptoms are often non-specific, with cognitive impairment, confusion, altered consciousness, headache, visual impairment and seizures as the most common symptoms. In severe cases, the condition can lead to coma and death (7).
Characteristic radiological findings are oedema in white matter, most frequently in the posterior (parieto-occipital) regions of the brain (5). In contrast to bilateral posterior infarctions, the calcarine and paramedian regions of the occipital lobe are often without oedema. MRI plays an important part in diagnosis (8), as the lesions have an enhanced signal on MRI images with T2 weighted and FLAIR sequences. These lesions usually have a high ADC value, an indicator of vasogenic oedema and hence reversibility, and thereby a better prognosis (9, 10). The use of susceptibility-weighted imaging (SWI, a relatively new method) has shown that microhaemorrhages are not unusual in the affected areas in patients with PRES, mainly in the cortex, but also in white matter (8).
There is debate as to whether the name is descriptive of the clinical and neuroradiological characteristics. Since the condition was first described in 1996 (5), several cases have been reported which show that the condition is not always reversible, and that the changes are not always located in white matter or in the posterior regions of the brain (11–13).
PRES is associated with an increasing number of medical conditions in which the syndrome may occur as a complication. The most common conditions are hypertension, (pre)eclampsia, renal failure, sepsis and autoimmune diseases. The syndrome may also develop directly during treatment with immunomodulatory or cytotoxic medications. The condition is a rare complication of angiogenesis-inhibitor therapy, but is documented in connection with treatment with sorafenib, sunitinib and pazopanib. However, a report on a connection between regorafenib and PRES has only been published in one previous case (14).
Regorafenib inhibits protein kinases involved in tumour angiogenesis, oncogenesis, metastasis and tumour immunity (15). The criterion for treatment is metastasised colorectal cancer in a patient who has previously been treated with fluoropyrimidine-based chemotherapy without satisfactory efficacy. In national guidelines for colon and rectal cancer, the drug is approved for use as a third-line therapy for patients in good general condition. It has been shown to increase median survival by 1.4 months compared with placebo, and is not recommended for routine use because of the limited efficacy (16).
The pathogenesis of PRES is still unclear, but the assumed mechanism is endothelial dysfunction following direct toxic effect on vascular endothelium (8). In cases where patients with high blood pressure develop the condition, the cause may be a greater increase in blood pressure than the cerebral autoregulation can manage. In these cases it is believed that high cerebral perfusion pressure with subsequent damage to the blood-brain barrier may cause vasodilation and hyperperfusion with subsequent vascular leakage and brain oedema (17). In our patient, the highest measured blood pressure was 149/91 mm Hg, so it was fairly unlikely that hypertension would be the cause. At present there is no consensus on specific treatment of PRES, only a symptomatic approach. Emphasis is placed on early identification and treatment of the underlying cause of the condition in order to avoid complications and permanent neurological deficit, or in the worst case, death due to the development of cytotoxic oedema, haemorrhaging and ischaemia (8, 10).
In many cases of PRES, immunosuppressive or cytotoxic drugs are identified as the cause of the neurological manifestations. The treatment strategy is therefore to discontinue the treatment. However, it is a matter of contention whether tapering or temporary discontinuation of the triggering drug is necessary, or whether a dose reduction may be sufficient (18, 19). Nor is there any consensus on a therapeutic approach once the neurological symptoms have regressed, but relapses are considered to occur very rarely. The prognosis is good, very often with normalisation in the course of a few days or weeks.
