Infertility affects about 10 % of all couples (1) and its incidence is increasing. The need for and use of fertility treatment is increasing in parallel, and the number of children conceived after fertility treatment has now surpassed eight million worldwide (2).
In Norway, assisted reproduction is defined by the Biotechnology Act, and includes techniques for both fertilisation of eggs outside the body and for insemination. Fertilisation of eggs outside the body can occur via in vitro fertilisation (IVF) or microinjection (intracytoplasmic sperm injection, ICSI) (3). Both techniques entail hormonal stimulation of the woman to induce controlled ovarian hyperstimulation for hormonal induction of egg maturation and simultaneous maturation of multiple eggs (Figure 1). Hormonal stimulation is usually performed with gonadotropins. Antioestrogens such as clomiphene citrate or letrozole are also used in certain cases. Although the former drug no longer has marketing authorisation in Norway, it may be prescribed under an exemption.
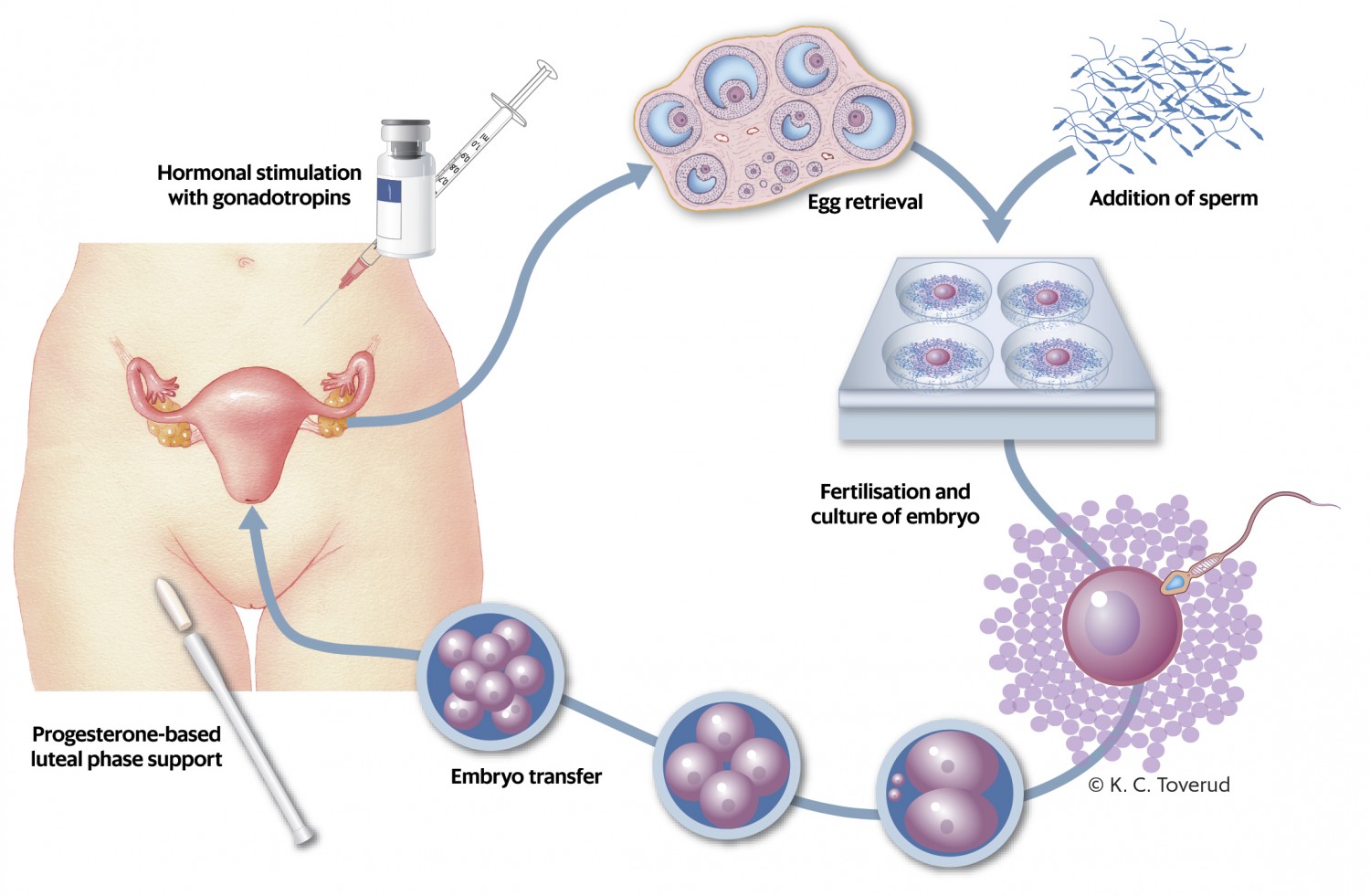
Figure 1 Fertility treatment with in vitro fertilisation.
The hormonal stimulation gives rise to brief periods of abnormally high levels of gonadotropins, oestrogen and progesterone (4). These hormones have previously been associated with an increased risk of several types of cancer (5, 6). Fertility treatment also involves puncture and aspiration of multiple follicles. This causes damage to the ovarian capsule and possibly cellular changes that increase the risk of subsequent cancer (7). This theory is supported by epidemiological studies showing that women with longer anovulatory periods have a lower risk of ovarian cancer (8). Children conceived through fertility treatment have in turn been shown to have increased risk of perinatal complications (9), congenital malformations (10), somatic disease (11) and cancer (12–14).
In this review, we summarise the evidence base for cancer risk in women after fertility treatment and in children conceived through fertility treatment.
Method
We searched the EMBASE and Medline databases with search strings for assisted fertilisation in combination with cancer (see appendix for search strategy and keywords). The search was terminated on 15 November 2017 and was limited to original English-language articles published after 1 January 2006. We included articles about women who had undergone fertility treatment with hormones and children conceived as a result of such treatment.
The search yielded 3 045 article titles, which were independently reviewed by all three authors (Figure 2). A total of 282 titles were considered relevant by one or more of the authors, and the abstracts for these articles were read by all. Sixty-four articles were then selected and read in full. Of these, the following articles were excluded: 22 because they were patient-control studies or did not address the issue in question, 14 because they concerned older types of treatment (1960s and 1970s), three because they did not compare risk in exposed versus unexposed individuals or used data that overlapped with those in later studies, and two because the type of exposure was not sufficiently specified. The reference lists from 11 reviews were also examined without yielding any further articles. In total, the review was based on 23 original articles. As the studies are heterogeneous, we did not perform any formal comparison or meta-analysis of all the data combined.
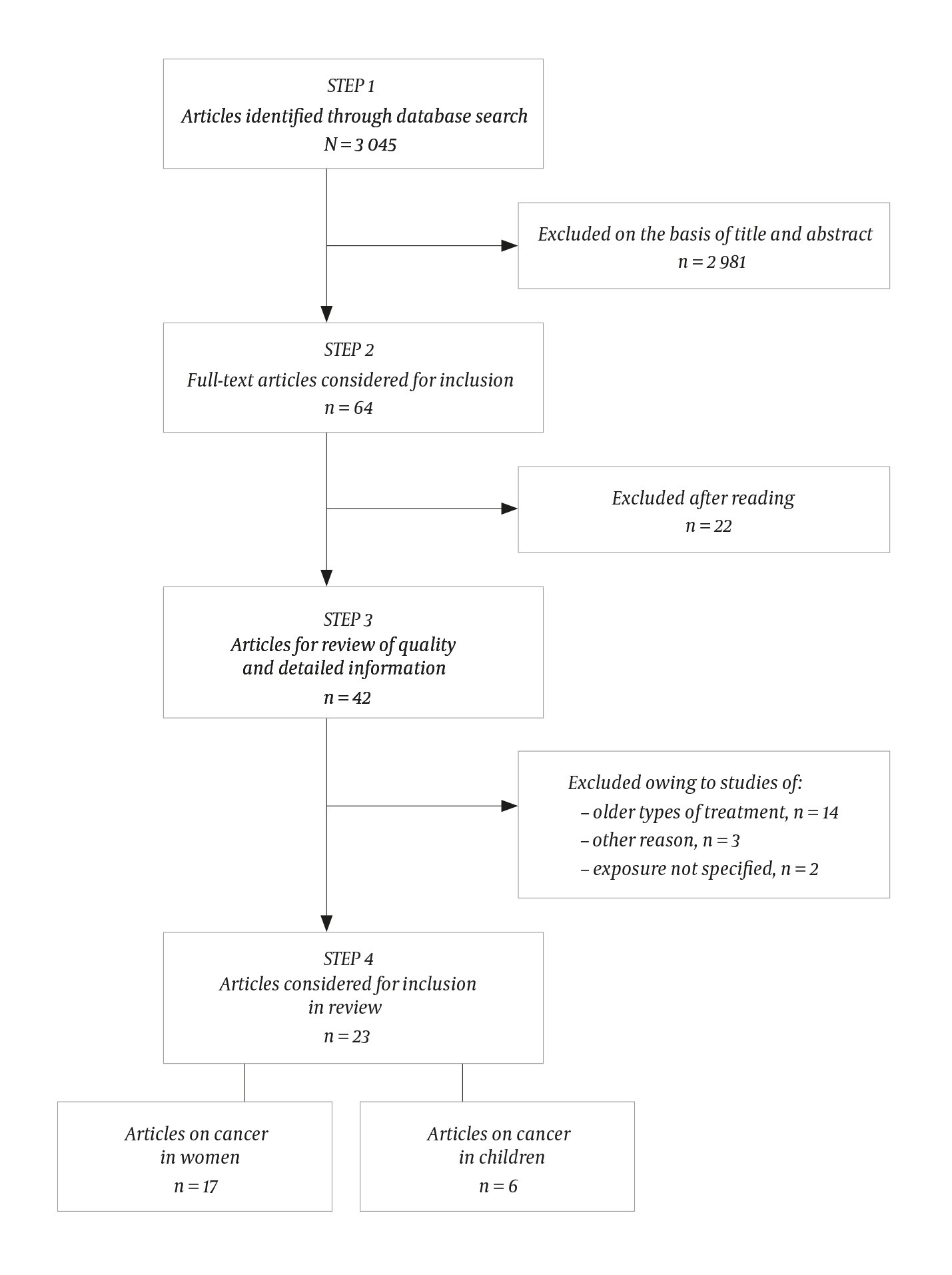
Figure 2 Flowchart summarising the literature search and the basis for selection of relevant cohort studies.
Results
A total of 17 cohort studies were included on cancer risk in women after fertility treatment. Tables 1–3 provide an overview of the studies on breast cancer (15–22), uterine cancer (19, 20, 22–24) and ovarian cancer (19–21, 25–28). Four studies examined overall cancer risk (19–21, 24), while three focused on other types of cancer (29–31). Six articles concerned cancer risk in children born as a result of fertility treatment (32–37); all examined overall cancer incidence, with five also reporting data for various types of cancer separately (Table 4).
Table 1
Cohort studies on breast cancer risk in women who have received fertility treatment. HR = hazard ratio, OR = odds ratio.
|
Study
|
Country
|
Number of cancer cases/ number exposed
|
Number of cancer cases/number unexposed
|
Risk (95 % CI)
|
Follow-up time, years
|
|
Lundberg, 2018 (18)
|
Sweden
|
262/38 047
|
13 152/1 302 164
|
HR 0.84 (0.74–0.95)
|
9.6 (mean)
|
|
Reigstad, 2017 (20)
|
Norway
|
112/31 675
|
6 578/1 322 049
|
HR 1.00 (0.81–1.22)
|
11 (median)
|
|
van den Belt-Dusebout, 2016 (15)
|
Netherlands
|
619/19 158
|
220/5 950
|
HR 1.01 (0.86–1.19)
|
21.1 (median)
|
|
Reigstad, 2015 (17)
|
Norway
|
138/16 626
|
7 899/792 208
|
HR 1.20 (1.01–1.42)1
|
16.0 (median)
|
|
Brinton, 2013 (22)
|
Israel
|
389/2
|
133/2
|
HR 0.90 (0.71–1.15)
|
8.1 (mean)
|
|
Yli-Kuha, 2012 (19)
|
Finland
|
55/9 175
|
60/9 175
|
OR 0.93 (0.62–1.40)
|
7.75 (mean)
|
|
Stewart, 2012 (16)
|
Australia
|
23/7 381
|
32/13 644
|
HR 1.10 (0.88–1.36)
|
16 (mean)
|
|
Källén, 2011 (21)
|
Sweden
|
91/24 058
|
13 492/1 394 061
|
OR 0.76 (0.62–0.94)
|
8.3 (mean)
|
Table 2
Cohort studies published since 2006 on uterine cancer risk in women who have received fertility treatment. HR = hazard ratio, OR = odds ratio.
|
Study
|
Year
|
Country
|
Number of cancer cases/ number exposed
|
Number of cancer cases/number unexposed
|
Risk (95 % CI)
|
Follow-up time, years
|
|
Reigstad (20)
|
2017
|
Norway
|
12/31 675
|
565/1 322 049
|
HR 0.76 (0.40–1.45)
|
11 (median)
|
|
Kessous (23)
|
2016
|
Israel
|
10/4 363
|
51/101 668
|
HR 4.6 (1.4–15.0)1
|
11.6 (mean)
|
|
Reigstad (17)
|
2015
|
Norway
|
5/16 525
|
626/789 723
|
HR 0.69 (0.28–1.68)
|
15.9 (median)
|
|
Brinton (22)
|
2013
|
Israel
|
34/2
|
7/2
|
HR 1.94 (0.73–5.12)
|
8.1 (mean)
|
|
Yli-Kuha (19)
|
2012
|
Finland
|
4/9 175
|
2/9 175
|
OR 2.0 (0.37–10.9)
|
7.75 (mean)
|
Table 3
Cohort studies published since 2006 on ovarian cancer risk in women who have received fertility treatment. HR = hazard ratio, OR = odds ratio.
|
Study
|
Year
|
Country
|
Number of cancer cases/number exposed
|
Number of cancer cases/number unexposed
|
Risk
(95 %
CI)
|
Follow-up time, years
|
|
Reigstad
(20)
|
2017
|
Norway
|
16/31 675
|
615/1 322 049
|
HR 1.62 (0.78–3.35)
|
11 (median)
|
|
Kessous (23)
|
2016
|
Israel
|
7/4 363
|
51/101 668
|
HR 3.9 (1.2–12.6)1
|
11.6 (mean)
|
|
Reigstad (17)
|
2015
|
Norway
|
16/16 525
|
800/789 723
|
HR 1.56 (0.94–2.60)
|
15.6 (median)
|
|
Brinton (22)
|
2013
|
Israel
|
34/1
|
11/2
|
HR 0.90 (0.45–1.79)
|
8.1 (mean)
|
|
Stewart (28)
|
2013
|
Australia
|
16/7 548
|
22/14 098
|
HR 1.36 (0.71–2.62)
|
17 (mean)
|
|
Trabert (26)
|
2013
|
USA
|
8/952
|
77/8 873
|
RR 1.0 (0.48–2.08)
|
21.9 (mean)
|
|
Yli-Kuha (19)
|
2012
|
Finland
|
9/9 175
|
3/9 175
|
OR 2.57
(0.69–9.63)
|
7.75 (mean)
|
|
Källén (21)
|
2011
|
Sweden
|
26/24 058
|
1 753/1 394 061
|
OR 2.09
(1.39–
3.12)1
|
8.3 (mean)
|
|
Van Leeuwen
(25)
|
2011
|
Netherlands
|
61/19 146
|
16/6 006
|
HR 1.14
(0.54–2.41)
|
14.7 (median)
|
Table 4
Cohort studies on cancer risk in children conceived through fertility treatment. HR = hazard ratio, RR = relative risk, SIR = standardised incidence rate, OR = odds ratio
|
Study
|
Country
|
Cancer diagnosis
|
Number of cancer cases/number exposed
|
Number of cancer cases/number unexposed
|
Risk (95 % CI)
|
Follow-up time, years
|
|
Wainstock, 2017 (33)
|
Israel
|
All diagnoses
|
7/2 603
|
415/237 863
|
HR 1.96 (1.14–3.36)1
|
10.5 (median)
|
|
Reigstad, 2016 (35)
|
Norway
|
All diagnoses
Leukaemia
Cancer of central nervous system
|
51/25 782
17/25 782
12/25 782
|
4 503/1 602 895
1 029/1 602 895
1 007/1 602 895
|
HR 1.21 (0.90–1.63)
HR 1.67 (1.02–2.73)1
HR 1.25
(0.71–2.21)
|
6.9 (median)
|
|
Lerner-Geva, 2017 (36)
|
Israel
|
All diagnoses
Leukaemia
Cancer of central nervous system
|
21/9 042
2/9 042
2/9 042
|
361/211 763
92/211 763
70/211 763
|
RR 1.18 (0.80–1.75)
RR 0.44 (0.14–1.40) RR 0.50 (0.20–1.24)
|
10.6 unexposed
9.3 exposed
(median)
|
|
Sundh, 2014 (34)
|
Nordic countries
|
All diagnoses
Leukaemia
Cancer of central nervous system
|
181/91 796
61/91 796
42/91 796
|
638/358 419
638/358 419
114/358 419
|
HR 1.08 (0.91–1.27)
HR 1.06 (0.80–1.41)
HR 1.44
(1.01–2.05)1
|
9.5 (mean)
|
|
Williams,
2013 (32)
|
United Kingdom
|
All diagnoses
Leukaemia
Cancer of central nervous system
|
108/106 013
34
22
|
109.7 (expected)
37.5 (expected)
25.8 (expected)
|
SIR 0.98 (0.81–1.19)
0.91 (0.63–1.27)
0.85 (0.54–1.29)
|
6.6 (mean)
|
|
Källén, 2010 (37)
|
Sweden
|
All diagnoses
Leukaemia
Cancer of central nervous system
|
53/26 692
18 (observed)
15 (observed)
|
6 405/2 417 878
12.3 (expected)
8.1 (expected)
|
OR 1.45 (1.10–1.91)1
2
2
|
Not stated
|
Breast cancer
Of eight cohort studies, five showed no association between fertility treatment and the risk of breast cancer (Table 1).
Studies from non-Nordic countries were based mainly on data from hospital records (15, 16, 18). None found an association between treatment and breast cancer risk. The studies conducted in the Nordic countries (17–21) used data from central population registries. Two Swedish studies found a significantly reduced risk of breast cancer (18, 21), whereas one Norwegian study found a significantly increased risk (17). The average follow-up time in the Swedish studies was 8.3–9.6 years, while in Norway, the median follow-up time was almost 16 years (17). The first of the Swedish studies (21) included information on exposure from all in vitro fertilisation clinics, information on all births from the Swedish Medical Birth Register and information about cancer diagnoses from the Swedish Cancer Registry. The more recent Swedish study (18) contained more detailed information about exposure from multiple registries.
One of the Norwegian studies, which used information on exposure from the Medical Birth Registry of Norway, found a significantly increased risk of breast cancer in women who became pregnant after fertility treatment (17). However, the most recent Norwegian study, which used exposure information from the Norwegian Prescription Database, found no increased risk of breast cancer in either parous or nulliparous women (20), with the exception of elevated risk in a subgroup of women who gave birth after treatment with clomiphene citrate.
Uterine cancer
Four of five cohort studies (Table 2) found no significant association between fertility treatment and uterine cancer (19, 20, 22, 24). However, an Israeli study (23) reported a significant five-fold increased risk. Subgroup analyses in the most recent Norwegian study (20) found an increased risk of uterine cancer in women exposed to more than six cycles of treatment with clomiphene citrate and in women who remained nulliparous after treatment.
Ovarian cancer
Seven of nine cohort studies (Table 3) showed no significant increase in the risk of ovarian cancer in women who received fertility treatment, while two reported significantly increased risk (21, 23). One of the studies found an increased risk of ovarian cancer after in vitro fertilisation but, surprisingly, the risk was even higher before these women underwent treatment (21). The authors believe this could be due to the women being at increased risk both infertility and of developing ovarian cancer (21). The highest risk estimates were in a study from Israel, where the risk of ovarian cancer was reported to be almost four times higher in women who had undergone fertility treatment and given birth at a particular medical centre (23). No significant association between fertility treatment and ovarian cancer was found in the two Norwegian studies, but subgroup analyses showed that women with primary infertility had significantly increased risk (24), as did women who were treated with clomiphene citrate alone or who remained nulliparous (20).
The risk of borderline ovarian tumours in women who had undergone fertility treatment was increased in four studies, including the Norwegian study (19, 20, 25, 27).
Other types of cancer
An Australian study showed no increase in the risk of malignant melanoma (29), and two studies from the Netherlands showed no increase in the risk of either malignant melanoma or colorectal cancer (30, 31). Four studies that examined overall cancer risk found no increase in association with fertility treatment (19–21, 24).
Paediatric cancer
Table 4 shows the results for all forms of paediatric cancer combined and for leukaemia and cancer of the central nervous system, which are the most common cancers in children. Two studies found an increased risk of all diagnoses combined (33, 37), while all studies showed significantly increased risk of one or more forms of cancer.
In Norway and Sweden, more cases of leukaemia were reported among children born after assisted conception than expected on the basis of population incidence (35, 37). However, no increased risk of leukaemia was found in Israel (36), the United Kingdom (32) or the other Nordic countries (34). Findings have been less consistent with regard to the risk of cancer of the central nervous system (34), hepatoblastoma and rhabdomyosarcoma (32), and retinoblastoma and renal tumours (36).
Discussion
Generally, fertility treatment cannot be said to increase the cancer risk in women and their children.
The current review includes studies from the period 2006–2017, owing to the fact that a number of new studies have been published since the last literature review (38). Treatment has also changed over time and, by restricting the time frame, we hoped to include studies with more homogeneous treatment exposure. Nevertheless, the studies proved to be relatively different in terms of methodology. Some studies took into account age, infertility diagnoses and the number of children borne by the women after treatment or whether they remained nulliparous. Other studies did not include such information. In addition, it is very important to control for overweight in connection with uterine cancer, which most studies did not.
A strength of the Nordic studies is that they used data from population-based registries, and also analysed the data at an individual level. A weakness of the Norwegian dataset is that there is no national, cycle-based registry of fertility treatment. Such a registry should be established. The Cancer Registry of Norway has been shown to have completeness of almost 100 % (39). However, quality and completeness are not as good in all countries (40). In the Israeli study (23), the authors included only those cancer cases diagnosed at the one centre from which the study population was recruited, a clear weakness of the study.
The Nordic cohort studies compared cancer risk in women who have undergone fertility treatment with the risk in the general population. Several of the non-Nordic studies obtained data from fertility clinics, which then enabled them to compare the risk of cancer in women who have had fertility treatment with the risk in untreated infertile women. In order to examine the effect of fertility treatment alone, the latter approach should ideally be used. However, this is difficult in practice, as the selection of women for treatment means that these two groups will differ. In addition, infertile or nulliparous women are a heterogeneous group, and various infertility diagnoses may themselves be associated with increased cancer risk. For example, endometriosis may be associated with increased risk of ovarian and uterine cancer (41, 42).
Other key challenges relate to study size and follow-up time. Many of the studies have few cancer cases in the exposed group, and several of the studies have short follow-up times. This is particularly relevant in the case of uterine cancer, which mainly affects older women (60–70 years of age).
Conclusion
There is no clear increase in cancer risk in women who undergo fertility treatment or in the children conceived as a result of such treatment.
As an increasing proportion of women undergo treatment for infertility, it is necessary to monitor the health of these women as well as the health of their children, also in terms of cancer risk.
Good quality, large cohort studies with longer follow-up times for both women and children are necessary.
