Exercise-induced rhabdomyolysis is a condition characterised by breakdown and necrosis of striated skeletal muscle cells, with systemic release of muscle enzymes such as creatine kinase (CK) and myoglobin. There is no absolute CK threshold for rhabdomyolysis, but CK levels > 5–10 times the reference level or CK levels > 5 000 IU/l in blood, together with muscle pain and a positive urine dipsticks test for blood are often used as diagnostic criteria (1). In Norway, the number of patients hospitalised with exercise-induced rhabdomyolysis is increasing (2–4). Pain is a major part of the clinical picture (5), and those who are hospitalised often have relatively little previous experience of the exercise that led to their admission (6–8). Damage to muscle cells renders the cell membrane permeable to muscle enzymes. This gives rise to moderately increased CK levels, whereas higher CK levels are thought to result from muscle necrosis (9).
The treatment of rhabdomyolysis is based on studies of trauma patients, often earthquake victims, and consists of large volumes of intravenous fluids in addition to other supportive treatment (10). It is well documented that this treatment prevents renal failure. On the other hand, it is resource-intensive and involves a risk of overhydration, electrolyte disturbances and infections. Patients with exercise-induced rhabdomyolysis are often treated using the same procedure, despite much better prognosis and lower risk of kidney injury (2, 7, 11–14). There is thus a risk of overtreatment as these patients will rarely benefit from this approach. To gain insights into normal physiological changes after intensive exercise, we examined levels of CK and creatinine in the blood, as well as the onset of haematuria, in healthy persons in association with an intensive workout. We also examined whether pain and previous exercise history were related to changes in CK levels.
Material and method
The project has been approved by the Regional Committee for Medical and Health Research Ethics (case number 2016/639). We invited medical students at NTNU to participate in the study through emails and information given after a lecture. The participants were required to have no known heart, lung or kidney disease, and to not be using statins. They were instructed to abstain from strength training for one week prior to the workout, and to abstain from all exercise for the three days leading up to the experiment. Thirty volunteers signed up, six of whom withdrew before the experiment. Twenty-four participants (14 women) performed the exercise intervention, completed questionnaires, and provided blood and urine samples. Written consent was obtained from all participants.
One high-intensity ‘Tabata’-type workout was performed, lasting approximately 50 minutes. The workout began with 10 minutes of general warm-up. Strength and cardiovascular training were then performed on many different muscle groups with high intensity and short breaks (20 seconds of activity, 10 seconds rest). Each exercise was repeated six times before a one-minute break and change of exercise, with eight exercises in total. Finally, a competition was held to stand against a wall for as long as possible with 90-degree hip and knee flexion (‘wall sit’). The participants were asked to drink plenty of fluids after the workout.
The day before and four days after the workout, blood and urine samples were taken to obtain baseline values and maximum values (9). Sampling and analysis of CK and creatinine in blood, as well as urine dipstick testing, were performed by the Department of Medical Biochemistry, St. Olavs Hospital, Trondheim University Hospital. Urine dipsticks positive for blood were used as an indirect index of myoglobinuria.
Before the workout, the participants completed a questionnaire on how often they engaged in strength training and on their regular medication use. On day four, the participants completed a questionnaire on muscle pain after the workout.
Statistical analyses were performed in SPSS Statistics version 25 (IBM SPSS Inc., Chicago, IL). A p-value < 0.05 was considered significant. Normally distributed data are presented as mean (standard deviation), non-normally distributed data as median (interquartile range). A student’s t-test for repeated measures was used for comparison of creatinine before and after exercise, and a Wilcoxon signed rank test for comparison of CK levels before and after exercise. Spearman’s rank correlation was used to analyse the association between previous exercise history, pain and CK increase.
All participants with CK levels > 5 000 IU/l after exercise were offered a follow-up measurement. Eight of 14 completed the follow-up, all of whom showed a decrease in CK levels.
Results
The median age of the participants was 24 (24–27) years. The pre-exercise blood sample haemolysed for one of the participants; baseline CK level could thus not be determined and this participant was excluded from analyses that include CK change. All participants showed an increase in CK levels, from a median of 104 (72–212) IU/l on the day before exercise to a median of 6 071 (2 815–12 275) IU/l on day four, p < 0.001 (Figure 1). Fourteen of 24 participants had CK levels > 5 000 IU/l. Four participants had urine dipsticks positive for blood on day 4. These four individuals had CK levels of 2 815, 5 248, 13 535 and 35 440 IU/l, respectively. Three of them thus had rhabdomyolysis according to the definition of muscle pain, CK > 5 000 and urine dipsticks positive for blood.
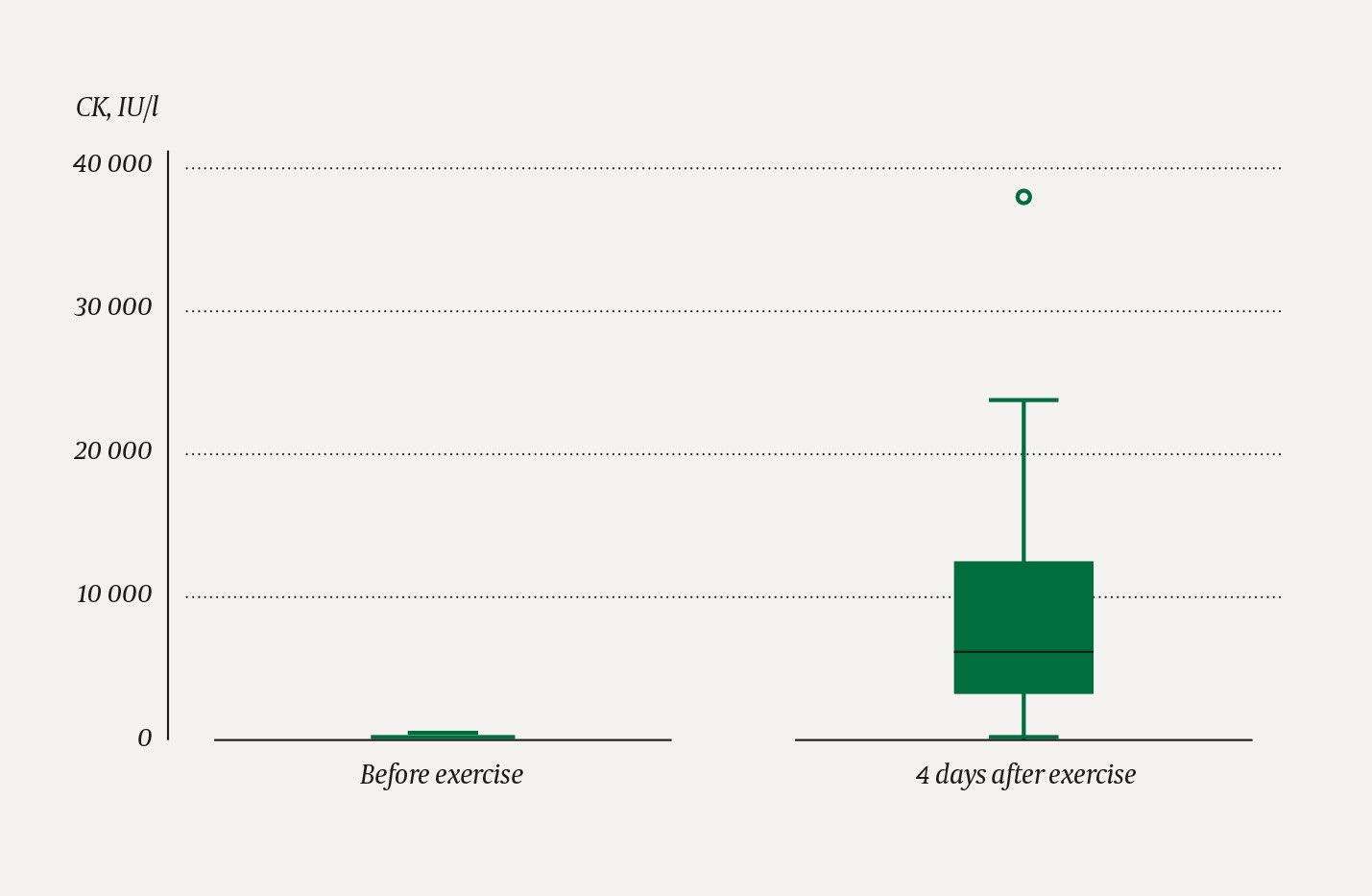
Figure 1 CK levels measured before exercise and on day 4 after exercise, in 24 healthy subjects. The median is represented by the line in the middle of the filled box. The box represents the 25–75 % interquartile range. The whiskers show the range of the data set; the circle shows an outlier.
Strength training history, as measured by the number of strength training sessions per week, showed a negative Spearman’s rank correlation with the CK increase, rho = -0.477 (p = 0.021). (Figure 2).
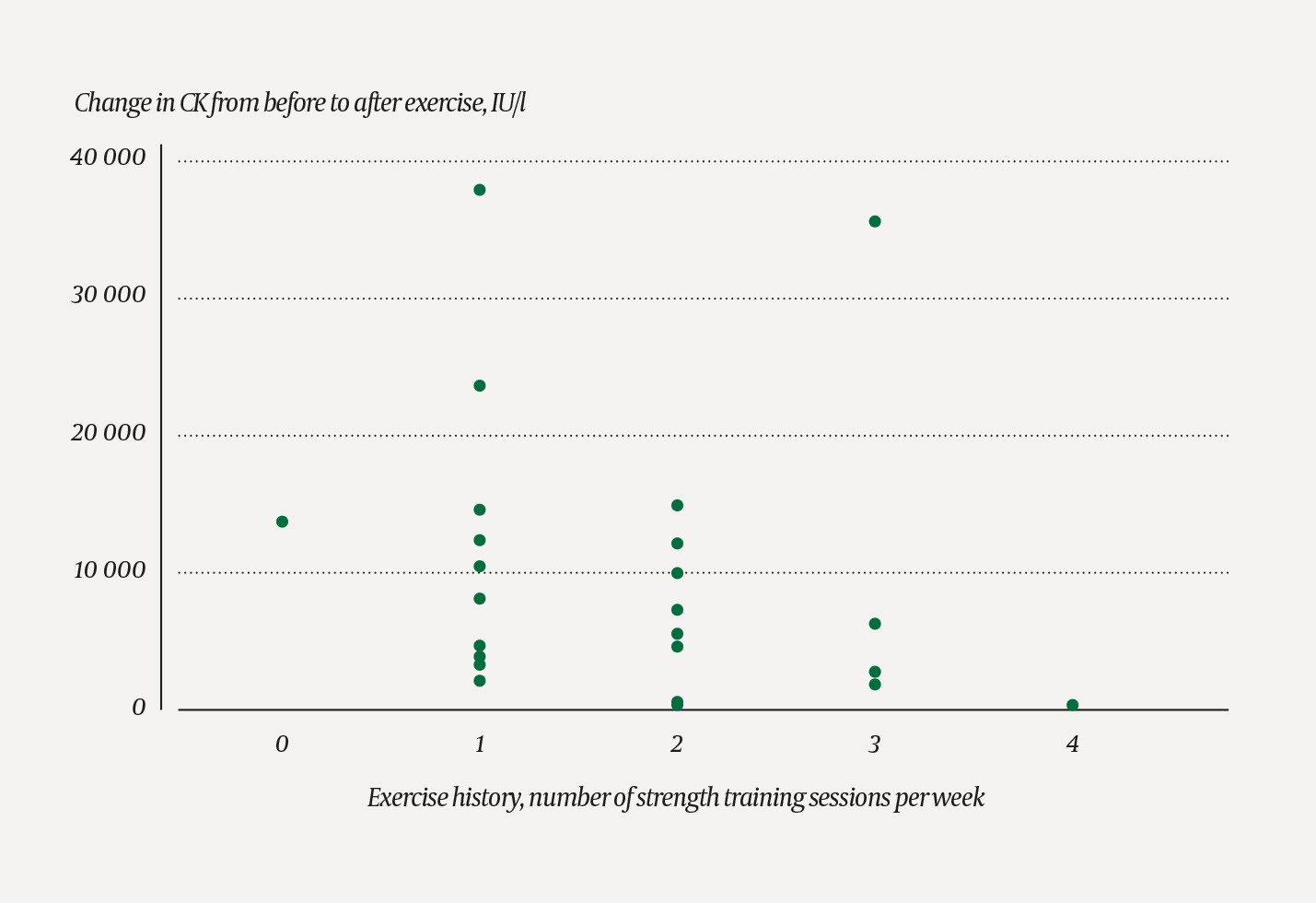
Figure 2 Scatter plot showing CK levels in 24 healthy subjects stratified according to number of reported strength training sessions per week. Significant negative Spearman’s rank correlation, rho = -0.347 (p = 0.097), with a smaller CK increase in participants who engage more frequently in strength training.
Creatinine levels were normally distributed and decreased from a mean of 69.4 (65.5–73.4) µmol/l the day before exercise to 67.0 (62.6–71.3) µmol/l on day four, p = 0.013. None of the three participants with rhabdomyolysis showed an increase in creatinine levels. We found no correlation between degree of self-reported muscle pain and the CK increase. Ten women were using hormonal contraceptives; only one participant used other regular medications.
Discussion
This experiment shows that intensive, varied exercise leads to a marked increase in CK levels, as previously demonstrated in studies using standardised exercise loads (12, 15). A marked increase in CK levels must thus be considered a normal phenomenon after an intensive workout. Moreover, we have shown that previous exercise history correlates negatively with the increase in CK levels. Since CK levels increased in all participants, there is reason to believe that most people with exercise-induced rhabdomyolysis are neither diagnosed nor treated in hospital.
Several risk factors for increased CK levels after exercise have been proposed in the literature: dehydration, overheating, high humidity, medication use, eccentric exercise and limited exercise history (1, 3, 6). It is possible that variables such as sex, age, genetics and ethnicity may also affect the CK increase, but our dataset is too small to allow subgroup analyses. Our participants were encouraged to drink plenty of fluids after the workout, and this most likely explains the statistically, but not clinically, significant fall in creatinine levels.
One weakness of the study is that participants were not restricted from exercising between the study workout and the sampling on day four. Exercise during this period may have led to a further CK increase in some participants. However, the finding of a significant CK increase remains unchanged, even though some participants may have engaged in additional exercise.
Larger clinical trials of patients hospitalised with exercise-induced rhabdomyolysis and treated with various hydration protocols are required to assess the risk of renal damage and determine the best treatment for this condition. We believe there is a need to reevaluate whether euvolaemic and healthy patients with exercise-induced rhabdomyolysis should receive the same treatment as patients with rhabdomyolysis attributable to other factors.