Here we describe a girl who attended hospital for routine pinnaplasty. Although there was nothing in the patient’s medical history to suggest an unusual reaction to anaesthesia, the child turned out to be completely unresponsive to opioids.
A previously healthy girl of early school-age attended hospital for elective surgery to correct protruding ears. The pre-operative anaesthesiology visit revealed nothing of note: She had no known allergies, no prior experience of anaesthesia, was healthy, used no regular medications and weighed 21 kg. She was classified as ASA I (healthy patient, assumed to be at minimal risk from anaesthesia based on defined criteria (1)). Total intravenous anaesthesia was planned with intubation, without the use of muscle relaxants and with local infiltration anaesthesia in the surgical area bilaterally. She received oral premedication of paracetamol 600 mg and midazolam 8 mg.
Intubation anaesthesia is the standard procedure in our hospital when patients are to undergo bilateral surgery to correct protruding ears. The position of the head changes during the procedure and there is limited access to the airways without interrupting the surgery. The procedure is suitable for local infiltration anaesthesia, and therefore long-acting opioids are not usually required postoperatively.
The mother accompanied the patient in to surgery. A Venflon intravenous catheter was inserted and connected to pre-programmed pumps with remifentanil (short-acting opioid) and propofol (intravenous anaesthetic). The girl had a pulse of 90 beats/min and O2 saturation of 100 % when anaesthesia was initiated. Remifentanil infusion was started at 0.3 µg/kg/min. Due to agitation/anxiety, the propofol infusion was started almost simultaneously with a pre-programmed bolus of 4 mg/kg followed by an initial maintenance dose of 12 mg/kg/h. The girl fell asleep quickly, and her mother was escorted out of the operating theatre. The patient experienced a brief apnoea, but promptly resumed spontaneous breathing. She was breathing at a rate of 20–26 breaths/min and with a tidal volume of 120–140 ml (almost normal for her age in an awake, calm patient). The remifentanil infusion was increased up to 1 µg/kg/min, an unexpectedly high dose. The Venflon, infusion pump and connectors were checked to rule out paravenous infusion, disconnection or misprogramming. After having received almost 4 μg/kg of remifentanil, the patient was still not showing the expected signs of an opioid response, and another anaesthesiologist (senior consultant) was summoned to the operating theatre.
It is well known that there is large interindividual variability in opioid response, but a dose of 4 μg/kg remifentanil over 7–8 minutes is 2–4 times that which would induce apnoea in the vast majority of opioid-naïve patients. In combination with propofol 4 mg/kg, this dose suppresses the protective pharyngeal reflex to such a degree that most patients can be intubated.
When the senior consultant arrived in the operating theatre, the patient was asleep but was breathing independently and had normal-sized pupils. Her heart rate was 93 bpm and blood pressure 100/60 mm Hg. Another series of checks was performed to rule out the most common sources of error that could potentially lead to an inadequate opioid effect, and a new syringe of remifentanil was ordered in case there was something wrong with the contents of the previous syringe. In the meantime, another opioid, fentanyl, was administered in repeated doses of up to 100 μg at short intervals, i.e. 4.8 μg/kg.
Remifentanil comes in vials as a dry substance that must be reconstituted. It has been known for syringes to be connected that contained no active drug (only NaCl). A completely new syringe was therefore mixed from a new vial. The relative potency of opioids varies in the literature. Fentanyl is often reported to be 80–100 times stronger than morphine per administered dose, i.e. 100 μg of fentanyl is equivalent to 8–10 mg of morphine intravenously.
Fentanyl had no effect either. The remifentanil syringe was therefore replaced with a new one. The patient continued to breathe evenly with about 20 breaths/min and an unchanged tidal volume, even with a total remifentanil dose of 8 µg/kg (170 µg) over the course of 15–20 minutes. A morphine syringe (2 mg) had been prepared in advance in case it should be required postoperatively. This was also administered without effect. A switch was therefore made to combined anaesthesia with gas. Sevoflurane (inhalation anaesthetic) was administered and the propofol infusion was stopped. By that point, the patient had received 150 mg propofol, or just over 7 mg/kg. A muscle relaxant (cisatracurium 3 mg intravenously, approximately 0.15 mg/kg) was administered, and with an end tidal sevoflurane concentration of 2.9 %, she was intubated. Her pulse increased to 140 bpm and blood pressure rose from 90/40 mm Hg to 118/67 mm Hg. Ketamine was then administered at 40 mg intravenously, and her pulse and blood pressure decreased again. The surgeon infiltrated the surgical sites with a local anaesthetic (bupivacaine 2.5 mg/ml with adrenaline). Anaesthesia was maintained with sevoflurane and ketamine, and the procedure was completed in just over two hours. A total of 100 mg ketamine was administered intravenously. Dexamethasone 0.3 mg/kg was also given intravenously as adjuvant analgesia and nausea prophylaxis. She received 10 mg of the NSAID ketorolac intravenously before awakening. The postoperative course was uncomplicated, with paracetamol and ibuprofen providing adequate pain relief.
Afterwards, there was discussion as to whether the girl’s opioid resistance could be due to genetic factors. The senior anaesthesiologist (author T.O.) spoke with the patient’s next of kin immediately after the surgery and explained the unexpected course. They were given practical solutions for providing pain relief postoperatively and over the next few days. They were also encouraged to contact our department should the patient be hospitalised for any other reason in the interim. The parents agreed that it was important to try to find an explanation for the events. They themselves had never experienced an unusual or absent opioid response, and asking their immediate family revealed that no-one else had experienced anything similar either.
After first contacting Oslo University Hospital, we later discussed the case with the head of research at the Department of Psychopharmacology, Diakonhjemmet Hospital (author E.M.), where a pharmacogenetic opioid panel had recently been established. The genetic analyses in this panel, however, were thought to be unable to explain the pronounced opioid resistance in our patient. This is because our patient’s opioid response was not reduced or altered, but instead appeared to be completely absent. A literature search revealed a recent publication from St. Olavs Hospital, Trondheim University Hospital, in which other variants of the μ-opioid receptor gene (OPRM1 (NM_000914.4)) had been presented (2). There was particular interest in a mutation (rs79910351, c.541C>T), which encodes an inactive (‘signalling dead’) μ-opioid receptor due to a change in the amino acid at position 181 of the protein (NP_000905.3, p.Arg181Cys). This mutation was functionally characterised by Ravindranathan et al. in 2009 (3). Laboratory reagents were ordered for analysis of this mutation in the Department of Psychopharmacology, and a blood sample was obtained from the patient in the outpatient clinic. The results revealed that the girl was a homozygous carrier of the variant in question.
Discovery of the homozygous Arg181Cys mutation explained the events during the girl’s surgery, in which she showed no response to potent opioids.
After genetic counselling, both parents were subsequently offered the opportunity to undergo pharmacogenetic analysis of the Arg181Cys mutation in OPRM1. Both mother and father were found to be heterozygous carriers. This means that their children will have a 25 % risk of being homozygous for the mutation.
Discussion
The girl remained asleep on propofol, and showed no observable response to the intravenous opioids administered. As this was an elective procedure, one might question whether the intervention ought not to have been aborted, given the complete absence of an opioid response. However, experience indicates that postoperative pain after the procedure in question can be managed successfully without opioids. With the routine use of long-acting local anaesthetic and with supplementary paracetamol and NSAIDs, pain is rarely an issue. Should any problems have arisen, preparations were in place for the patient to remain in the observation unit for the first 24 hours. On this basis, it was decided to proceed with the operation.
It soon became clear that the patient lacked a normal opioid response. The pure mu (μ) agonists remifentanil and fentanyl had no effect, even in large doses. It was suspected even at this stage that there might be abnormalities at the receptor level. Morphine also acts to some degree via the kappa (κ) and delta (δ) receptors, and it is possible that higher doses of morphine might have had an effect. The analgesic effects of opioids are mediated mainly via μ-opioid receptors, and μ-receptor-mediated intracellular signalling is G-protein coupled. Previously, μ-opioid receptors were subdivided into μ1 and μ2 receptors, and it was thought that opioid analgesia was mediated by μ1 stimulation, whereas many of the adverse effects were the result of μ2 stimulation. This has subsequently been refuted. The general consensus now is that there is only one type of μ-opioid receptor, and that subtyping of opioid receptors should be avoided (4, 5).
The gene for the μ-opioid receptor, OPRM1, exhibits significant polymorphism. This is thought to partly explain the large variation in the clinical effects of opioids (6). Individual differences in uptake, metabolism and transport across the blood-brain barrier also contribute substantially to between-patient differences in clinical opioid response (7). In our patient, on the other hand, it was an inactivating mutation in the OPRM1 gene that was responsible. The mutation in question had been described previously by Skorpen et al. in Trondheim (2), and leads to a change in the amino acid at position 181 from arginine to cysteine (2). This amino acid is located in the intracellular domain of the receptor, and the mutation prevents transduction of extracellular stimulation into an intracellular response (Figure 1). Homozygous carriers of this mutation, such as the girl in this case report, will therefore not respond clinically to the opioids that are most commonly used in anaesthesia, such as fentanyl, alfentanil and remifentanil. Other opioids will also have no effect on pain, unless they additionally stimulate kappa and delta receptors.
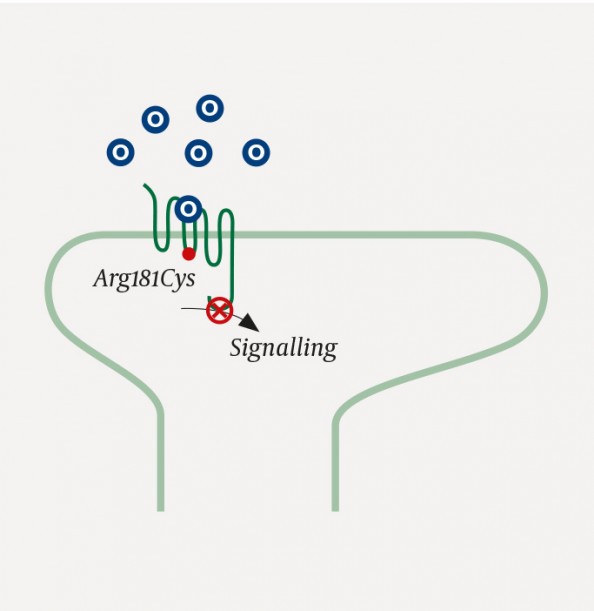
Figure 1 An opioid receptor with an Arg181Cys mutation. Despite proper binding of opioids (O) to the μ-opioid receptor, intracellular signal transduction is not triggered. The receptor is inactive (‘signalling dead’) (2).
The OPRM1 mutation that had been homozygously inherited by the girl in this case report, has an allele frequency of 0.25 % in the European population (8) and approximately 1/200 (0.5 %) in the Norwegian population, see figures published by Skorpen et al. (2). This means that the frequency of homozygous carriers of the Arg181Cys mutation in OPRM1 is about 1 in 40 000 (2). Being completely unresponsive to opioids as a result of this mutation is therefore rare, but of critical significance. Skorpen et al. also demonstrated a decreased opioid response in heterozygous carriers of the Arg181Cys mutation. Heterozygous carriers comprise about 1 % of the population, and will therefore regularly be encountered by clinicians in the context of pain management.
A pharmacogenetic panel for investigation of the opioid response, which incorporates both OPRM1 (including the Arg181Cys mutation) and relevant CYP enzymes involved in opioid metabolism, is now available as a routine analysis at the Department of Psychopharmacology, Diakonhjemmet Hospital. To what extent patients should be screened for the mutation in clinical practice is an issue for discussion in the field. As a basic principle, it would be sensible to consider pharmacogenetic testing of patients with a very unusual opioid response following discussion with specialists in pain management. An important point in this context is that patients with long-term use of opioids for chronic pain, such as cancer patients, may develop strong opioid tolerance that is unrelated to mutation of the OPRM1 gene. Skorpen et al. have shown that an increased frequency of the Arg181Cys mutation may be anticipated in patient groups with a poor opioid response. Further research is required to determine how often pharmacogenetic variation may account for an inadequate opioid response.
The pharmacogenetic analysis of OPRM1 explained the absence of a therapeutic response to opioids in our patient. The results of the genetic testing are also important for the future: the girl in this case report will encounter challenges should she require acute pain relief later in life. The use of opioids is such an integral part of the acute treatment of moderate to severe pain that patients may be subjected to a trial and error approach to achieve pain relief, unless clinicians are aware that they fully lack an opioid response. One of the aims of the new Norwegian ‘summary care record’ is for this type of critical information to be available irrespective of which hospital in Norway the patient is admitted to. However, this requires doctors to manually enter information into the system. As of yet, there is no functionality to link information from the summary care record (e.g. genotype) to prescriptions, in a way that would enable automatic alerts to be generated to assist with clinical decision-making.
Patients who are homozygous for the Arg181Cys mutation in OPRM1 will require personalised pain management. Prior to any surgery, the surgeon and anaesthesiologist should agree on a strategy for perioperative management and post-operative pain relief. Infiltration anaesthesia and peripheral or central blocks will be helpful where these are possible. A plan should be in place for managing breakthrough pain and any failure of a blockade. In addition to basic analgesia with paracetamol and/or NSAIDs, adjuvant agents such as corticosteroids and clonidine may be indicated. For stronger pain, ketamine in combination with a benzodiazepine or small doses of propofol would be a natural choice. In hospitals, the use of intravenous ketamine is often limited in practice to specialist wards. For acute painful conditions that would normally require opioids, it is probably reasonable for these patients to initially be placed under observation with close follow-up by an anaesthesiologist or others with special expertise in pain management.
