A woman in her fifties with known hypertension and paroxysmal atrial fibrillation was admitted to hospital somnolent and hypotensive. ECG showed a broad complex cardiac rhythm with QRS width greater than 300 ms.
A woman in her fifties was acutely hospitalised after being found unresponsive by relatives. She had been taking medication for hypertension and paroxysmal atrial fibrillation for several years. She had no family history of sudden cardiac death. Two weeks prior to the hospital admission, she had undergone cryoablation for paroxysmal atrial fibrillation with pulmonary vein isolation. Pre-surgical echocardiography showed normal systolic function, but diastolic dysfunction, grade 1 mitral insufficiency and enlarged atria. Following the ablation, prophylaxis was initiated with flecainide tablets. While on treatment with flecainide, the woman experienced postoperative recurrence of rapid atrial fibrillation accompanied by a fall in blood pressure. Flecainide was therefore discontinued, and she was cardioverted to restore sinus rhythm. Seven days prior to the current admission, she was discharged from hospital with beta blockers as her only medication.
At the time of the current admission, the patient was somnolent and hypotensive with blood pressure of 86/56 mm Hg. Her pulse was 73 beats/min and regular. Peripheral oxygen saturation was 95 % without supplemental oxygen. Clinical examination of the heart and lungs was normal. She showed spontaneous movement of all extremities, but was unable to cooperate with a full neurological examination. Arterial blood gas analysis showed pH 7.34 (reference range 7.36–7.44), pCO2 4.3 kPa (4.5–6.1 kPa), pO2 9.5 kPa (11.0–13.0 kPa), HCO3 17 mmol/l (22–26 mmol/l), base excess −7.5 mmol/l (0 ± 3 mmol/l) and lactate 3.5 mmol/l (0.4–1.3 mmol/l). Venous blood samples showed leukocytes 21.3 ∙ 109/l (3.5–11.0 ∙ 109/l), glucose 14.1 mmol/l (4.0–6.0 mmol/l), CRP 6 mg/l (<5 mg/l), creatinine 94 µmol/l (45–90 µmol/l), gamma glutamyltransferase 169 U/l (10–75 U/l) and hs-troponin T (TnT) 11 ng/l (<15 ng/l). Coagulation status, electrolytes and other liver enzymes were within reference ranges. Electrocardiography (ECG) showed a broad complex rhythm with QRS width of 380 ms (<100 ms), corrected QT interval (QTc) according to Bazett’s formula of approximately 640 ms (<440 ms) and global ST-T wave changes (Figure 1).

Figure 1 ECG from day 1 shows a broad complex rhythm with QRS width greater than 380 ms and diffuse ST-T wave changes. Heart rate 73 beats/min. Corrected QT interval approximately 640 ms.
Following examination in Acute Admissions, and in light of the patient’s relatives describing an emotional stressor prior to the incident, the woman’s condition with somnolence, hypotension and broad complex cardiac rhythm was considered to reflect possible intoxication. She had no fever or stiffness of the neck, and respiration was unaffected. Other causes of somnolence and circulatory failure such as sepsis, meningitis, a cerebrovascular event, pulmonary embolism or myocardial infarction were considered unlikely.
The patient was intubated to perform gastric lavage, administer activated charcoal and prevent aspiration. No remains of tablets were observed in the gastric aspirate. She was transferred to the intensive care and observation unit, and 500 mmol sodium bicarbonate and 5 mmol calcium chloride were administered intravenously, with transient narrowing of the QRS complex. Treatment with vasoactive and inotropic drugs was started in addition because of haemodynamic instability. Echocardiography showed globally reduced left ventricular systolic function with an estimated ejection fraction (EF) of 30–40 % (>50 %). The patient’s relatives had discovered drug packaging suggesting that she might have taken 4–5 g flecainide, 0.3 g oxazepam and 0.5 g meclozine approximately five hours prior to being hospitalised. A suicide attempt was considered the most likely explanation, with the flecainide overdose the most serious component.
The treatment of acute flecainide intoxication follows standard guidelines for stabilisation of the airway and circulation, along with administration of activated charcoal to reduce gastrointestinal absorption (1, 2). The earlier activated charcoal is administered, the greater its efficacy, but it should not be given to persons with decreased consciousness unless the airway is protected, e.g. via intubation, because of the risk of aspiration (1). Gastric lavage may be considered, but recent studies suggest that the risk of complications may outweigh the benefits (3).
Flecainide is used in the treatment and prophylaxis of paroxysmal atrial fibrillation and other supraventricular arrhythmias. According to the Norwegian Pharmaceutical Product Compendium, there have been few overdoses, but 1.5 g is reported to have caused serious poisoning. Early symptoms of intoxication are dizziness, nausea and vomiting, followed by visual disturbances. Cardiac arrhythmias such as bradyarrhythmias, asystole, torsades de pointes ventricular tachycardia and ventricular fibrillation may occur. In addition, flecainide has a cardiodepressant effect, and impaired systolic function is frequently observed (4). There is no antidote, but narrowing of QRS complexes has been described upon treatment with sodium bicarbonate (5). Haemodialysis and haemofiltration have not been shown to be effective (6).
The following day, another dose of activated charcoal was administered upon advice from the Poison Information Centre owing to the possibility of delayed gastrointestinal absorption. ECG rhythms showed bradycardia, widened QRS complexes, multifocal ventricular extrasystoles, and runs of non-sustained ventricular tachycardia.
The patient had persistent hypotension and treatment with adrenaline and noradrenaline was continued. An attempt was made to stabilise the cardiac rhythm with 30 mmol magnesium sulphate without notable effect. As the patient still had arrhythmia and was hypotensive, a temporary pacemaker was implanted in the right ventricle with a lower frequency set to 80 impulses per minute. The ventricular threshold was measured at 0.5–1.0 mA. The patient quickly became haemodynamically stable, and adrenaline and noradrenaline were discontinued. Fever, yellow sputum and a CRP increase raised suspicion of aspiration pneumonia, and treatment with piperacillin/tazobactam was therefore started.
On day 2, echocardiography showed an ejection fraction of 50 %. ECG showed sinus bradycardia with QRS width of 92 ms, corrected QT interval of 440 ms, and T-wave inversions in the standard leads (II, III, aVF) and the precordial leads (V3–V6).
On day 4, the temporary pacemaker was removed. The patient was extubated in the morning, but her systolic blood pressure rose over the course of the day to 200–220 mm Hg, and she developed presumed hypertensive pulmonary oedema. She had pronounced nausea, and metoclopramide and ondansetron were administered. She was reintubated in the evening, and that night experienced a recurrence of rapid atrial fibrillation. This was treated with amiodarone infusion.
On day 5, the atrial fibrillation was cardioverted to normal frequency sinus rhythm. ECG in sinus rhythm showed T-wave inversions in the standard and precordial leads, as previously, but an increasing corrected QT interval of 540 ms and narrow QRS complexes (97 ms). That same evening, the patient developed torsades de pointes ventricular tachycardia with a fall in blood pressure. A temporary pacemaker was again implanted in the right ventricle (lower frequency set to 110 pulses per minute), with a threshold of 0.5–1.0 mA, and 30 mmol magnesium sulphate was administered. Metoclopramide, ondansetron and piperacillin/tazobactam were discontinued as these drugs may contribute to QT interval prolongation. Electrolytes, creatinine and acid-base status were within reference ranges.
Possible causes of QT interval prolongation include metabolic abnormalities such as hypokalaemia and hypomagnesaemia, as well as combination therapy with other drugs that prolong the QT interval or that inhibit the breakdown of drugs that do so (7). Flecainide also prolongs the QT interval, but secondary to widening of the QRS complex (8). However, drug-induced QT prolongation is usually characterised by a prolonged corrected QT interval, but normal QRS width.
Magnesium sulphate has been shown to be effective in ventricular arrhythmias associated with a long QT interval or torsades de pointes tachycardia, but is not recommended as prophylactic antiarrhythmic treatment in cases of acute myocardial infarction (9). In our patient, magnesium sulphate did not have a stabilising effect when administered upon hospital admission, as flecainide increases the QRS width but not the QT interval (8). When, on day 5, the patient developed torsades de pointes tachycardia and a prolonged QT interval, probably drug-induced, we therefore chose to administer magnesium sulphate again, this time with a possible stabilising effect.
On day 6, the patient was tracheostomised due to an anticipated requirement for prolonged mechanical ventilation. She was successfully decannulated on day 9. She became haemodynamically stable, and the temporary pacemaker was removed. The corrected QT interval normalised, with almost complete normalisation of previous T-wave inversions in the standard leads. The results of flecainide concentration measurements showed that the patient had had very severe flecainide intoxication upon admission (Figure 2).

Figure 2 Flecainide concentration after ingestion. (The reference range for normal dosing is 500–2 400 nmol/l.)
On day 11, the woman again became increasingly dyspnoeic, but without accompanying chest pain. Auscultation revealed crackles over both lungs upon inspiration. ECG showed sinus rhythm, QRS width 97 ms, corrected QT interval 590 ms and a new-onset T-wave inversion in precordial leads V2–6 in addition to standard leads I, II, aVL and aVF (Figure 3). Over the course of the day, she had repeated episodes of rapid atrial fibrillation that were treated with beta blockers. Loop diuretics were administered owing to clinical signs of heart failure.
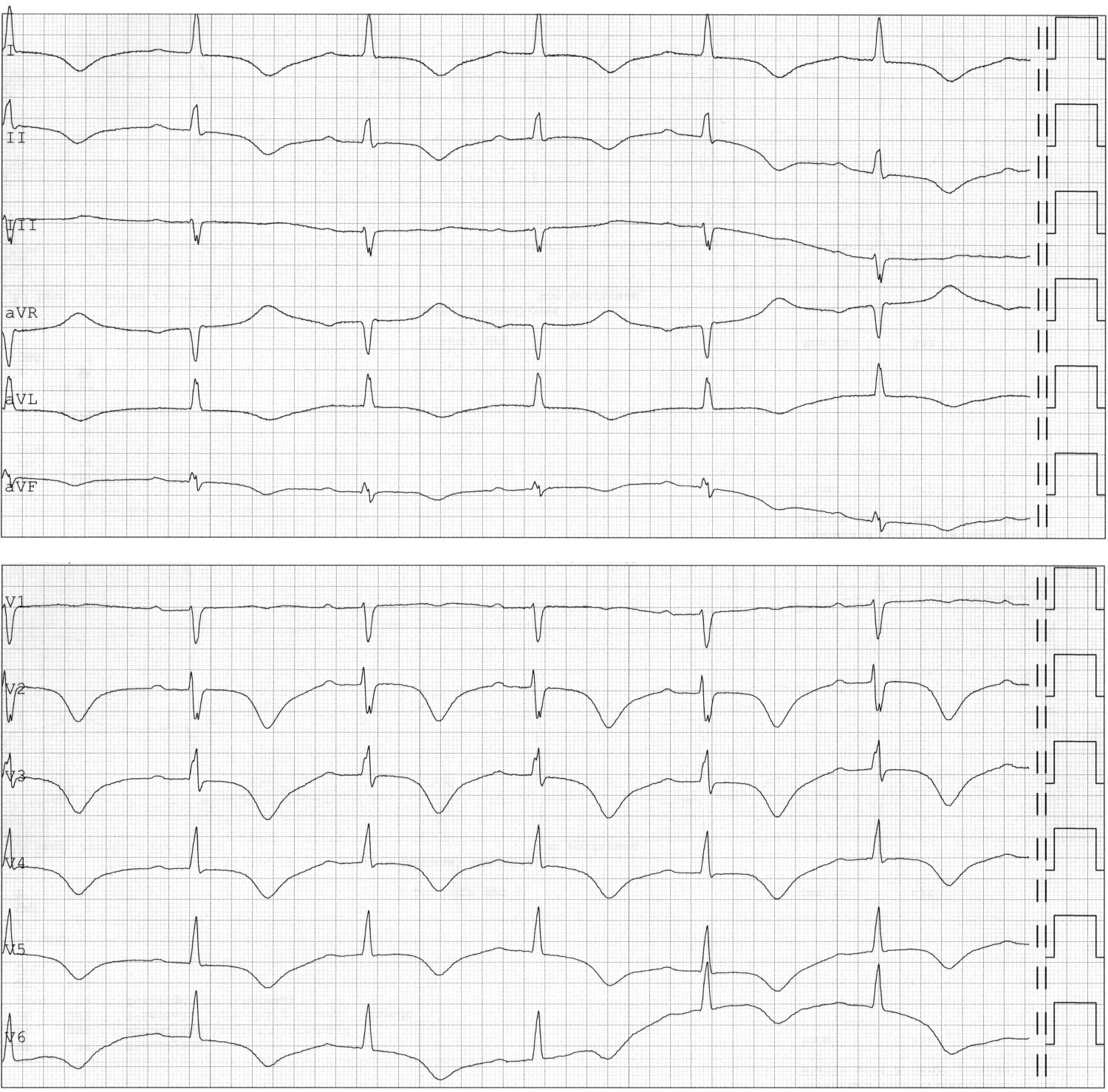
Figure 3 ECG from day 11 showing sinus rhythm but new-onset T-wave inversion in standard leads I, II, avL and aVF and in precordial leads V2–6. Corrected QT interval 590 ms.
On day 12, a chest x-ray showed signs of oedema and bilateral pleural effusion. Echocardiography revealed a normal-sized left ventricle, but now with hypokinesis and apical ballooning. The ejection fraction was reduced to 30–35 %, and pulmonary artery systolic pressure was estimated to be 60 mm Hg (<35 mm Hg). Ramipril treatment was started owing to hypertension with blood pressure of 158/100 mm Hg and heart failure. Serum hs-troponin T was 19 ng/l (< 15) and serum proBNP 8 567 ng/l (< 338 ng/l) on day 13.
Repeat ECGs on days 12 and 13 showed no ST elevations and the woman had no chest pain. During her hospitalisation for cryoablation a few weeks prior to the current incident, a CT coronary angiogram had been performed, which showed open coronary arteries. It was decided there was no indication for another cardiac investigation. The findings from clinical examinations, ECG and echocardiography could be consistent with stress-induced cardiomyopathy (Takotsubo syndrome).
On day 14, the patient was haemodynamically stable and in clinical recovery. Cardiac MRI showed findings consistent with stress-induced cardiomyopathy with apical hypokinesis and ballooning without signs of myocardial contrast uptake. ECG prior to discharge showed sinus rhythm, sustained deep T-wave inversions in standard leads I, II, III and aVF, and in precordial leads V2–6. The corrected QT interval was 550 ms and QRS width 103 ms.
The patient was discharged on day 17 in good health with psychiatric follow-up. She was tested in the Department of Medical Genetics for possible hereditary long QT syndrome, but with negative results. At a cardiology outpatient appointment three months after hospitalisation, the patient was in good health. ECG showed sinus rhythm and T-wave inversions in I, aVL and V4–6, and a normal corrected QT interval. Echocardiography showed a normal shape of the left ventricle, ejection fraction of 56 % and unchanged diastolic dysfunction with elevated filling pressure and a dilated left atrium.
Discussion
Flecainide is a class Ic antiarrhythmic agent that blocks sodium channels in cardiomyocytes, contributing to prolongation of the PQ interval and increased QRS width (8). Its half-life is normally given as 14 hours, but may be as long as 20 hours in the elderly. Flecainide is mainly excreted via the kidneys, 90 % in the form of inactive metabolites (10). Flecainide has high bioavailability (95 %), a large volume of distribution (6–8 l/kg) and moderate protein binding (40 %) (10).
For haemodialysis to work, the molecule in question must be able to pass through the filter (dependent on molecular size), show low protein binding, and have a low volume of distribution. Haemodialysis is therefore not an effective treatment for flecainide intoxication as these conditions are not met.
The cardiac arrhythmia suppression trial (CAST) showed increased mortality in myocardial infarction patients in whom attempts were made to suppress asymptomatic or mildly symptomatic ventricular ectopy with flecainide (11). Flecainide is contraindicated in cases of ischaemic heart failure, previous ventricular arrhythmias, left ventricular hypertrophy and atrioventricular block.
The woman in this case report had recurrent supraventricular arrhythmias, occasional self-limiting ventricular arrhythmias and a prolonged QT interval during parts of her hospital stay. It was considered whether delayed absorption of flecainide from the gastrointestinal tract might help explain the arrhythmias, but the patient’s flecainide concentration was falling throughout her time in hospital (Figure 2). Stress-induced cardiomyopathy was unlikely to be the cause of the cardiac arrhythmias since most of the arrhythmias occurred before day 11, when she showed a marked deterioration with symptoms of heart failure and clinical findings consistent with stress-induced cardiomyopathy.
In cases of flecainide intoxication, electrolyte and acid-base disturbances must be corrected. Treatment with sodium bicarbonate is used to increase the serum intracellular sodium concentration and raise the pH above 7.50 (6). The mechanism of action of sodium bicarbonate has not been definitively identified. A direct effect on the interaction between flecainide and sodium channels has been described, and alkalisation results in flecainide dissociating more rapidly from sodium receptors (12). One study showed that sodium modulates the binding of flecainide to the sodium receptor. It helps to increase conduction velocity by up to 33 % and to reduce QRS width (13). In cases of severe intoxication, repeated dosing (50–100 mmol/l) or continuous infusion of sodium bicarbonate has been attempted in an effort to raise the pH above 7.50 and the serum sodium concentration above 150 mmol/l (14). Treatment with 500 mmol sodium bicarbonate was initiated in Acute Admissions after ECG showed broad QRS complexes, with a beneficial effect on QRS width. We have subsequently considered whether repeated doses, or possibly continuous infusion, ought to have been used while the patient remained hypotensive during ongoing treatment with noradrenaline, and as neither the recommended treatment targets specified by a review article and several case reports (pH > 7.50, serum Na > 150 mmol/l), nor narrowing of the QRS complexes and stabilisation of the cardiac rhythm, had been achieved (4, 6).
Temporary transvenous cardiac pacing may be considered in cases of severe bradyarrhythmia. There is one previous report of a case of flecainide intoxication with persistent third-degree atrioventricular block in which a permanent pacemaker was implanted (15). A high pacing threshold and requirement for high impulse strengths have been described in cases of flecainide poisoning (16). In our patient, a temporary pacemaker was implanted on day 1 owing to persistent bradycardia and hypotension during ongoing treatment with vasoactive and inotropic drugs. Haemodynamic function was rapidly normalised after implantation of the pacemaker, and adrenaline and noradrenaline were discontinued.
Lipid emulsion therapy is reported to result in normalisation of heart rate, blood pressure and QRS width (17). The lipid emulsion binds lipophilic drugs; by binding to flecainide, it is hypothesised to prevent flecainide from binding to sodium channel receptors (18). However, the evidence base is highly uncertain. Venoarterial extracorporeal membrane oxygenation (VA-ECMO) may be considered for patients with flecainide poisoning with circulatory collapse in the absence of other severe comorbidities. The goal is to achieve adequate organ perfusion and thereby increase the metabolism and excretion of flecainide in the liver and kidneys (19). There are reports of single patients with treatment-refractory, life-threatening flecainide intoxication who survived following treatment with VA-ECMO (20). Lipid emulsion therapy was discussed for our patient, but VA-ECMO was also being considered should the patient develop biventricular heart failure. We decided against this therapeutic option owing to lack of experience with the use of lipid emulsion combined with VA-ECMO.
Conclusion
Flecainide intoxication is rare but potentially life-threatening. Acute treatment follows standard guidelines with stabilisation of the airway and circulation, and assessment of the indication for activated charcoal. The evidence base for specific treatment is limited, but treatment with sodium bicarbonate, a temporary pacemaker and possibly lipid emulsion should be considered on an ongoing basis. In very rare cases with persistent circulatory collapse, mechanical circulatory support may be appropriate.