The soreness that commonly follows unaccustomed and strenuous exercise is unlikely to be due to inflammation of the muscles. However, the rarer and more serious exercise-induced rhabdomyolysis appears to have a different pathogenesis, with clinical signs including tissue inflammation and muscle cell death, as well as elevated creatine kinase and myoglobinuria. Soreness and rhabdomyolysis can both be caused by the same type of muscular activity.
Muscle soreness is a very common skeletal muscle phenomenon with distinctive characteristics (1, 2; see Table 1). It occurs after unaccustomed, and especially eccentric, muscular activity – such as descending a mountain. The soreness peaks after 2–3 days, but seldom lasts more than a week. The muscles are particularly sore as one begins to move – for example, when rising from a chair – but little or no pain is felt when the muscles are completely relaxed. Movement, such as warming up for a sporting activity, will gradually reduce the feeling of soreness, but it will return again after the activity. When soreness is present, the muscles may feel as though they are weak and uncoordinated. While this can be the case, muscle function usually proves to be approximately normal (>90 %) when measured objectively with performance tests. A number of explanatory models for muscle soreness have been proposed, including lactate accumulation and spasms, but the most commonly given explanation is that muscle soreness, also known as delayed onset muscle soreness (DOMS), is due to cell damage and inflammation in the muscles (1–3). We will use only the term ‘muscle soreness’ throughout this article.
Table 1
Differences between muscle soreness and exercise-induced rhabdomyolysis (2, 3, 5, 20, 21, 24, 27–32). Arrows (↑↓) indicate an increase/decrease (graded 1–4), and parentheses show individual variation.
|
Mechanism, signs and symptoms
|
Muscle soreness
|
Rhabdomyolysis
|
|
Precipitating muscle action
|
1. Unaccustomed movements
2. Eccentric > isometric > concentric contractions
3. Large range of motion/muscle lengthening
|
1. The same as for muscle soreness, but tending towards extreme exercise for the individual, major exertion and/or amount
2. Prolonged reduction in blood flow/ischaemia may be a mechanism
3. High muscle/core body temperature, dehydration and hyponatraemia may reduce threshold
|
|
Latency of signs and symptoms
|
~8–12 hours
|
Muscle function: immediate and sustained reduction
Myoglobin and CK ≥ 12 hours
|
|
Most intense signs and symptoms
|
2–3 days
|
Urine/myoglobinuria: 1–3 days
Blood markers: 2–7 days
Tissue inflammation: 4–12 days
|
|
Duration/normalisation
|
4–7 days
|
> 3–4 weeks
|
|
Muscle soreness upon movement
|
↑(↑↑↑)
|
↑(↑↑↑)
|
|
Muscle tenderness to palpation
|
↑(↑↑↑)
|
↑(↑↑↑)
|
|
Resting pain
|
None
|
↑(↑↑↑) Possible inflammatory pain
|
|
Muscle swelling
|
None or little, but swelling due to muscle damage may possibly exacerbate the soreness.
|
↑(↑↑↑) Depends on the muscles affected and the degree of muscle damage. Risk of compartment syndrome must always be addressed.
|
|
Muscle stiffness and reduced range of motion/contracture
|
None, but stiffness due to muscle damage can probably exacerbate the soreness.
|
↑(↑↑↑)
|
|
Muscle function/strength
|
↓ Possibly as a result of reduced ability to activate the muscles.
|
↓↓(↓↓) Over 50 % reduction in maximum power. Damage to contractile and force transmission structures.
|
|
Laboratory blood tests
|
|
|
|
|
Myoglobin and CK
|
None
|
↑↑(↑↑) CK: ~5000–>100 000 IU/l
|
|
|
AST, ALT, LDH, uric acid, neutrophil gelatinase-associated lipocalin (NGAL)
|
None
|
↑(↑↑)
|
|
|
Creatinine, K+, CRP, cytokines (e.g. interleukin-6)
|
None
|
(↑↑)
|
|
Therapy
|
None. No intervention necessary, but massage and ice baths can reduce soreness.
|
Hospitalisation and intravenous saline, bicarbonate and crystalloid may be indicated if there is a possibility of renal damage. Physical rehabilitation 1–2 weeks after normalisation of signs and symptoms.
|
|
Clinical comments
|
The discomfort is usually manageable, but may be frightening. Important to recognise that soreness is a phenomenon that can coexist with muscle injuries and their symptoms.
|
Symptoms and clinical signs vary greatly, but myoglobin and CK in the blood are critical markers, as is myoglobinuria. Circulatory status should be clarified if there is severe swelling. Muscle function should be assessed to confirm full recovery on follow-up. Rehabilitation can take weeks to months.
|
In this clinical review, we propose an alternative mechanism for the pain associated with muscle soreness and compare it to the mechanisms underlying muscle injuries and rhabdomyolysis. This is based on our own experience of research in the field, as well as recent cellular and molecular studies in animal models selected from our personal archives and identified through unstructured searches.
Inflammation in cases of muscle soreness?
Muscular activity, particularly unaccustomed, eccentric muscle actions (stretching of contracted muscles), can lead to damage to myofibrils and sarcomeres (2, 4–6). The damage is visible under the electron microscope immediately after the muscular activity, but may become more extensive over subsequent days. In rare cases, it may take many weeks for the muscles to regenerate (see rhabdomyolysis below). Structural damage to the contractile apparatus, cytoskeleton and cell membrane leads to both reduced muscle function and apparently a local sterile inflammation (2; see Figure 1). Human studies of radiolabelled neutrophilic granulocytes, and the detection of these cells and of monocytes/macrophages in biopsies of stressed muscle tissue, have confirmed that an inflammatory response may accompany muscle soreness. Leukocytes may be present inside capillaries and between muscle cells, while macrophages may sometimes be found inside the muscle cells (7–9).
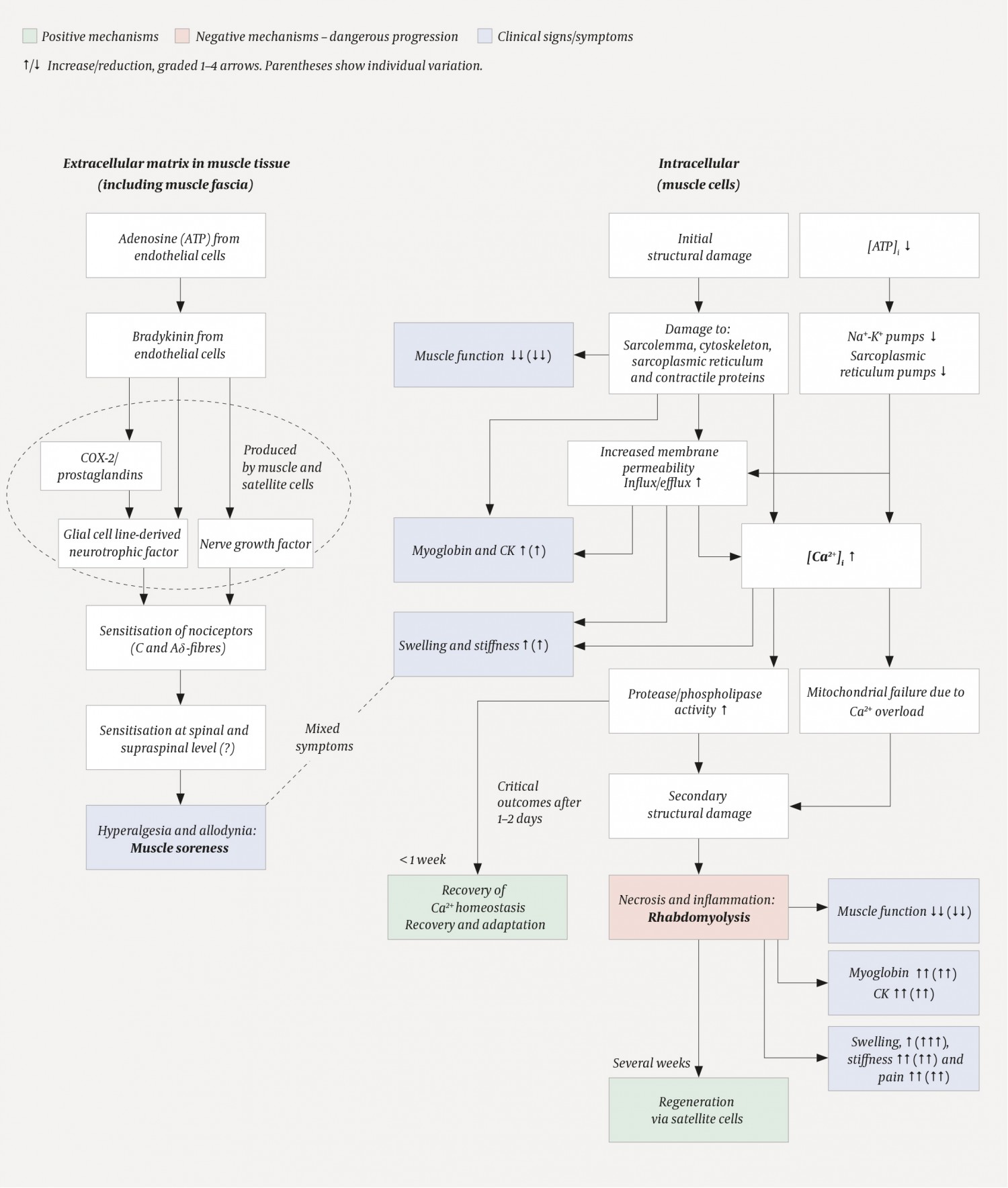
Figure 1 Schematic showing the putative course of events in muscle soreness and activity-induced rhabdomyolysis (2, 3, 5, 21, 32). The mechanisms underlying muscle soreness (left) are thought to be physiological processes in the extracellular space, whereas rhabdomyolysis (right) involves pathological intracellular processes. The dotted circle on the left is to show that COX-2/prostaglandins, glial cell line-derived neurotrophic factor and nerve growth factor are produced by muscle and satellite cells. i = intracellular.
Notably, however, the presence of leukocytes and muscle soreness did not follow the same time course. Leukocytes were first detected in the muscle tissue 48 hours after activity, whereas muscle soreness was already well-established after 24 hours – and was often in decline when levels of inflammatory cells in the muscle tissue were highest, i.e. 4–7 days after activity. In addition, it was possible for subjects with severe soreness to have very few or no signs of an inflammatory response in the muscles, and for others with strong signs of inflammation to report little soreness (7, 8). Moreover, no causal relationship has been established between muscle soreness and ‘classic’ cytokines such as interleukin-6 and TNF-α in human studies (2).
In a study (7) in which subjects were given a COX-2 inhibitor (celecoxib), muscle soreness was reduced but the drug did not affect the accumulation of inflammatory cells. Since celecoxib also had no effect on prostaglandin levels in the muscle interstitial fluid (measured by microdialysis), it is possible that it suppresses soreness via direct effects on the peripheral or central nervous system.
Animal models have provided evidence regarding the mechanisms underlying muscle soreness. Mizumura & Taguchi (3) summarise a number of studies performed on rats, which have approximately the same time course of soreness as humans. Rats cannot report how sore they are, but it is possible to measure how much mechanical pressure must be applied to the muscles before a rat withdraws its leg. This method has been validated in several ways, but it is important to remember that animal models can be misleading.
Mizumura & Taguchi (3) propose that muscle soreness is initiated by the formation of bradykinin (Figure 1). This vasodilatory polypeptide is a known inflammatory mediator and can activate nociceptors. Bradykinin is released during muscular activity and binds to the B2 bradykinin receptor present on muscle cells. This bradykinin activity stimulates increased synthesis of nerve growth factor (NGF) mRNA as well as ensuing protein synthesis, which is thought to occur inside muscle and satellite cells (muscle stem cells). The time required to produce nerve growth factor could potentially explain the delayed onset of muscle soreness. The growth factor can sensitise C-fibres and give rise to pressure hyperalgesia, which is typical of muscle soreness. However, bradykinin and nerve growth factor do not appear to be alone in causing soreness. Increased presence of glial cell line-derived neurotrophic factor (GDNF) induced by prostaglandin E2 upon stimulation of COX-2 activity may also contribute to hyperalgesia (Aδ fibres). The nociceptors that mediate muscle soreness in response to nerve growth factor and glial cell line-derived neurotrophic factor, thus appear to be the standard C and Aδ fibres. Since both bradykinin and prostaglandin E2 can be produced locally in the muscle and have autocrine and paracrine effects, muscle soreness does not appear to be dependent on inflammatory cells. This lends support to the results of the human studies described above: muscle soreness is not normally due to classic tissue inflammation.
It is worth noting that the processes described occur in the vicinity of capillaries and nerve endings in the extracellular matrix and connective tissue – not intracellularly, even though a number of the mediators are produced there (Figure 1). We therefore believe that muscle soreness is not directly related to intracellular muscle damage. However, we cannot exclude the possibility that some form of damage to the muscle connective tissue contributes to muscle soreness, as suggested by Abraham back in 1977 (10).
More than a local muscle reaction?
According to the hypothesis above (3), the soreness is thus a form of hyperalgesia. That is, the pain response is upregulated: firm pressure applied to the muscle will be perceived as more uncomfortable and painful than usual. Strong evidence that this is due to sensitisation of nociceptors (C and Aδ fibres) (3) does not rule out additional mechanisms at higher levels of the nervous system, e.g. in the spinal cord, the periaqueductal grey (PAG) or the thalamus (11, 12). This central sensitisation may be particularly relevant in rare cases where the soreness lasts more than 4–5 days.
The pain of muscle soreness may also be regarded as allodynia, because the nociceptors may respond to mechanical stimuli that do not normally cause pain or discomfort, e.g. light pressure or stretching of the muscles. An unconfirmed hypothesis states that mechanosensitive nerves, such as Aβ fibres from muscle spindles, stimulate ‘pain pathways’ at spinal cord level and cause allodynia (5). Allodynia may also be due to activity in nociceptive C and Aδ fibres, as it has been shown that these fibres can be stimulated by non-painful pressure and stretching of the muscles (3, 13). Just as the pain of fibromyalgia probably entails sensitisation of the dorsal horn of the spinal cord, so a similar mechanism may feasibly be involved in muscle soreness (12, 14).
How to provide relief?
It is reasonable to believe that soreness may be a signal that the muscles are in need of rest and relaxation, i.e. recovery. However, if this is the case, it is not a particularly reliable mechanism as it is possible to be in pain even without muscle function being notably reduced (8). On the other hand, soreness is usually, but not always, present when the muscles do in fact need rest. Thus, soreness has high sensitivity, but low specificity as a marker for muscle damage and the need for recovery. For top athletes, this would not be sufficient in any case, and they should therefore measure muscle function to determine when extra recovery time is required.
The only sure way to avoid soreness is careful, progressive training in exercises that would cause soreness if started at high intensity. A number of other measures have been attempted in an effort to reduce soreness that is already present. Many of these have no or negligible effect (1, 15), while others, such as repeated ice baths and massage can reduce soreness to some extent (15). Much of the soothing effect is short-lived and the soreness quickly returns – suggesting that the relief may reflect a temporary inhibition of the nervous system. There are no recognised drug therapies (16), but it is possible that the prophylactic use of non-steroidal anti-inflammatory drugs (NSAIDs), especially COX-2 inhibitors, just before exercise may be effective (7). On the other hand, both NSAIDs and ice baths can adversely affect muscle recovery and adaptation processes, such that reduced muscle soreness may come at the expense of reduced exercise benefits (2, 16, 17). It is unclear whether the mechanism that suppresses muscle soreness is the same mechanism that inhibits adaptation to exercise.
Rhabdomyolysis
In Norway, the incidence of rhabdomyolysis after exercise has recently been increasing (18–20). This seems to be due to the increased popularity of very intensive forms of exercise. Exercise-induced muscle damage can encompass everything from minimal subcellular damage, as occurs during regular exercise, to the breakdown of entire muscle fibres, i.e. necrosis and rhabdomyolysis.
We have observed the development of rhabdomyolysis in a number of subjects in our studies (4, 7–9), and have had the opportunity to follow the process closely both with functional tests and muscle biopsies (Figure 1, Table 1). The first sign is an immediate and significant reduction in muscle function following muscular activity, to less than 50 % of maximum strength. The initial damage includes disruption of the regulatory mechanisms of Ca2+ homeostasis (2, 5, 6), i.e. ion channels and pumps in the sarcoplasmic reticulum. This increases resting levels of calcium ions in the muscle cell, which results in increased activity of various proteases, e.g. those of the calpain system, as well as phospholipases (2, 21). The proteases exacerbate the intracellular damage by breaking down the ‘support beams’ of the cytoskeleton (including desmin and dystrophin). If the cytoskeleton collapses, the muscle cell membrane will tear. Increased cell membrane permeability results not only in an uncontrolled release of intracellular proteins such as creatine kinase (CK), but also a further increase in Ca2+ levels (influx). Areas or segments of the muscle cell become caught up in a vicious circle and die – muscle fibres are rarely damaged along their entire length (2). The necrotic process initiates a powerful inflammatory response and subsequent regeneration. Signs of necrosis can be seen after about 48 hours, while the inflammatory response peaks after about one week. Assuming an intact basement membrane, activation of satellite cells (stem cells) and good circulation, the regeneration will continue for a number of weeks (2). The muscle thus continues its recovery and regeneration processes long after the soreness has disappeared.
Rhabdomyolysis is diagnosed by measuring CK and myoglobin levels in the blood (22). These measures are important as a high myoglobin load can cause renal failure. Treatment, for example with intravenous saline, crystalloid and bicarbonate, must be considered if CK levels are above 5000 IU/l or five times the upper limit of normal. Rapid initiation of treatment has proved crucial in severe cases (21, 23, 24). Typical signs and symptoms are severe muscle pain and swelling, as well as muscle weakness, stiffness and reduced range of motion (Figure 1, Table 1). An important difference between commonplace muscle soreness and rhabdomyolysis is that the latter also gives rise to muscle pain at rest. However, it should be emphasised that even extreme pain does not necessarily have to mean rhabdomyolysis. Myoglobinuria, by contrast, is a sure sign of muscle damage, but not necessarily an index of the severity of the condition (19, 24).
Certain genetic backgrounds appear to predispose individuals to rhabdomyolysis (24, 25), but the most important factor is how one exercises. Particular care is required when initiating exercise regimes that involve substantial eccentric muscle contractions. It is a common misconception that muscles must be damaged to become bigger and stronger (26).
Summary and conclusion
Muscle soreness is a form of hyperalgesia and allodynia. The mechanism(s) appear(s) to be independent of damage to muscle fibres and classic tissue inflammation. The main difference between muscle soreness and rhabdomyolysis is that soreness should be considered a physiological phenomenon localised to extracellular structures, the muscle fascia and the nervous system (sensitisation), whereas rhabdomyolysis is an intracellular pathological condition of the muscle cells. Muscle soreness and rhabdomyolysis may occur in parallel in response to muscle overload (summarised in Figure 1 and Table 1).
A doctor consulted by a patient complaining of muscle pain should, with the help of the anamnesis and palpation of the muscle, be able to determine whether the pain reflects soreness or the more dangerous rhabdomyolysis. Urinalysis and analysis of myoglobin and CK in the blood will reveal whether hospitalisation of a patient with rhabdomyolysis is indicated.
