Patients in intensive care have increased nutritional needs but are often incapable of eating independently. When should intravenous parenteral nutrition be started, and what is the optimal dose? Here we review the recently updated European guidelines on nutritional support in intensive care patients.
During critical illness, the body is in a hypercatabolic state in which muscle mass and energy stores are depleted and nutrients are used at a high rate (1). This catabolic state manifests clinically as weight loss, sarcopenia and undernutrition. This has been shown to prolong stays in intensive care, delay recovery, increase complications and, most worryingly, increase mortality (2). Proper nutritional support is of greater importance for these patients than many clinicians appreciate, and undernutrition or overnutrition, as well as incorrect choices with respect to initiation and route of administration, may be crucial in determining clinical outcomes.
The European Society for Clinical Nutrition and Metabolism (ESPEN) has recently published updated guidelines for the use of nutritional support in intensive care patients (3), and these differ on several points from the previous version that was last updated in 2009 (4). European guidelines still recommend starting parenteral nutrition earlier and at higher doses compared to the American recommendations, which are published by the American Society for Parenteral and Enteral Nutrition (ASPEN) (5).
The new guidelines provide far greater scope for personal judgement, which presupposes a good knowledge base among clinicians and familiarity with the considerations in the different guidelines. In this article, we provide a brief overview of key points in the recently updated European guidelines, and review the most important changes, which concern the initiation and route of administration of medical nutrition. The main points are summarised in Box 1.
Box 1 Selected ESPEN recommendations for nutritional support in intensive care patients (3).
The risk of malnutrition should be assessed in all patients admitted to intensive care units.
Medical nutrition should be considered for all intensive care patients with an anticipated stay of more than 48 hours.
Oral intake should be attempted first. If this is insufficient, enteral feeding via nasogastric tube should be started within 48 hours.
Hypocaloric nutrition (< 70 % of the patient’s daily caloric needs) is recommended for the first 48 hours, followed by isocaloric nutrition.
Prokinetic drugs should be used in the event of poor tolerance of enteral feeding via nasogastric tube. Consider whether post-pyloric jejunal feeding is indicated.
If enteral feeding is insufficient to meet daily caloric needs, initiation of parenteral nutrition is recommended on the 3rd–7th day after admission to intensive care.
Intensive care patients should receive doses of nutrition in accordance with their individual needs as calculated via indirect calorimetry.
Hyperglycaemia (> 10 mmol/l) during nutritional support should be treated with insulin, and blood sugar levels should be kept between 6 and 8 mmol/l.
Metabolic changes in critical illness
Seen from an evolutionary perspective, catabolism in the critically ill is a survival response. The underlying mechanisms are complex, but are initiated by stimuli from stress hormones such as cortisol and inflammatory factors that trigger metabolic changes in various organs (Figure 1) (1). Glucose, which can be readily converted into energy, is quickly consumed along with glycogen stores in the liver. The body then releases amino acids and other metabolites for the regeneration of glucose, and uses released metabolites to repair tissues and strengthen the immune system. The nutritional needs of critical organs such as the glucose-dependent central nervous system are prioritised at the expense of tissue as muscle. The body enters a state of negative protein balance and a temporary but strong insulin resistance arises in the muscles. This is most pronounced clinically over the first seven days. This period is often referred to as the metabolic acute phase, and the first 48 hours as the early acute phase. Following the acute phase, the patient enters a late phase with a transition to anabolic metabolism in which energy stores and tissues are built up, but in seriously ill patients, the catabolic phase may develop into a chronic phase that can last for several weeks.
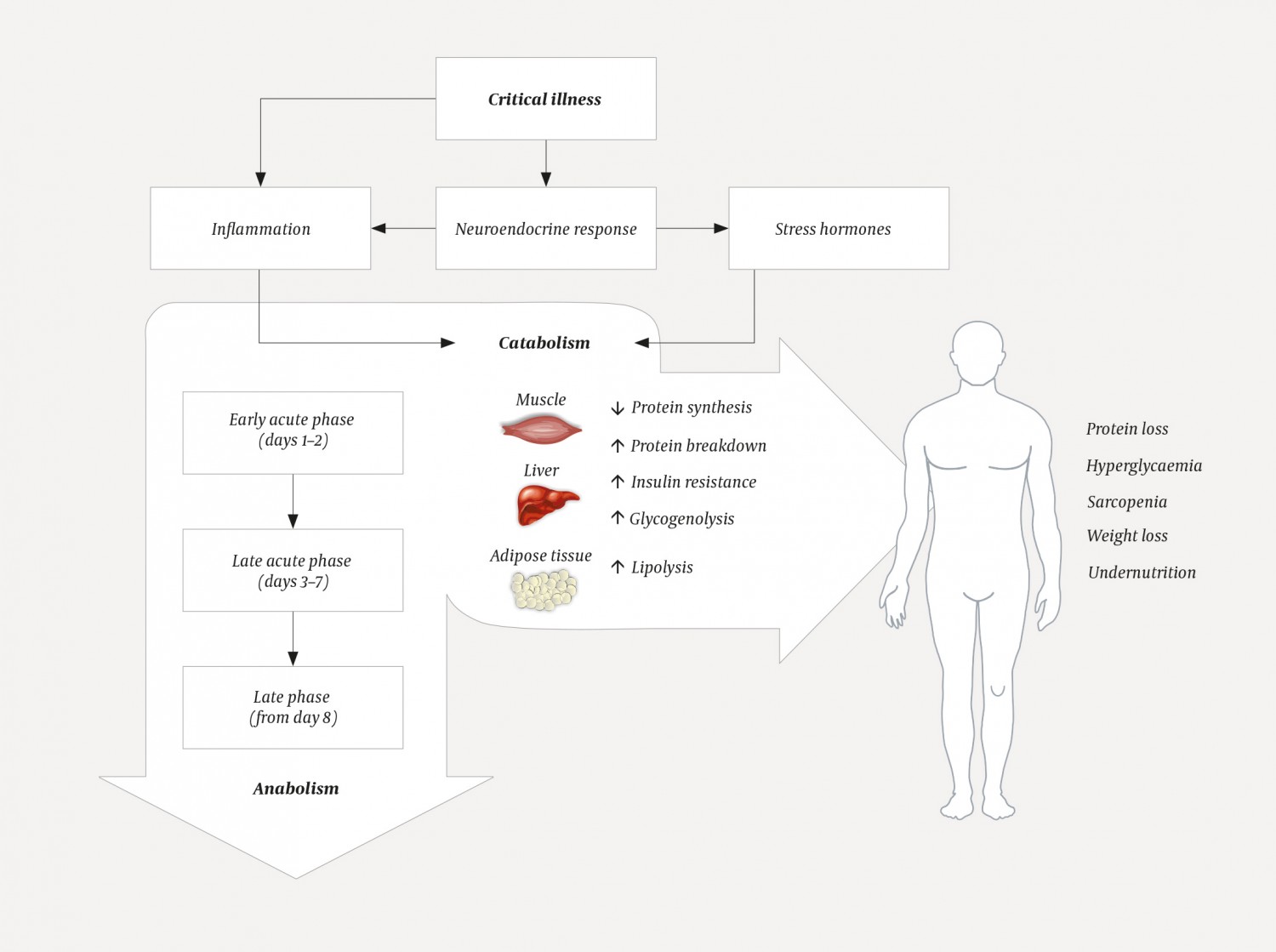
Figure 1 Critical illness gives rise to a neuroendocrine and inflammatory response that initiates a variety of metabolic changes, especially in insulin-sensitive tissues such as muscle, liver and adipose tissue. The response manifests clinically in the early acute phase and gives rise to a strong catabolic response in the delayed acute phase, before the patient enters a more anabolic late phase.
Nutritional screening
The risk of malnutrition should be evaluated in all intensive care patients in the early acute phase, as early parenteral nutrition is recommended only for those patients with severe undernutrition. The challenge lies in the choice of tools. Weight changes and body mass index (BMI) are important to consider, but are of little use alone. Biochemical parameters such as albumin, subjective assessment of the patient’s capacity for nutritional intake, and disease severity are often used, but should in fact play little part in the clinical assessment (6). Radiological determination of muscle and fat mass is more accurate but too resource intensive for routine use. Screening tools in the form of scoresheets have therefore been developed. Several of these are recommended (6), including NRS-2002, which is used by most Norwegian hospitals. No scoresheet has yet been validated for use in intensive care patients. Although the American guidelines explicitly recommend the use of NRS-2002, the European guidelines include only a general recommendation for the early assessment of nutritional status.
Enteral nutrition
Intensive care patients are a very heterogeneous population, and specific recommendations are available for trauma, liver failure and pancreatitis patients, among others. These recommendations will not be discussed here, but practitioners are encouraged to familiarise themselves with them when appropriate (3, 5). Nutritional support should only be postponed in unstable patients with conditions such as septic shock, haemorrhage or intestinal ischaemia. All other intensive care patients with an anticipated hospital stay of more than 48 hours should receive medical nutrition in the early acute phase (3, 5).
Oral intake is always preferable, but provides inadequate nutrition in up to 80 % of intensive care patients owing to the nature of the disease, ongoing sedation and/or intubation (3). In such cases, initiation of enteral feeding via nasogastric tube is recommended within at most 48 hours. Enteral feeding should be attempted prior to parenteral nutrition, provided there are no clear contraindications such as a high aspiration risk, or following certain surgical procedures. If the patient is intolerant of enteral feeding even at low doses, prokinetic drugs to increase intestinal mobility should be tried. Intravenous erythromycin is considered the first-choice treatment, with metoclopramide as an alternative or in combination.
If the patient is poorly tolerant of gastric administration, post-pyloric feeding via nasojejunal tube may be attempted. This reduces the risk of aspiration in predisposed patients (7). However, the results of large randomised controlled trials have failed to establish unambiguously whether a post-pyloric tube is in fact more effective at administering nutrition (2). Therefore, neither the European nor the American guidelines explicitly recommend trying post-pyloric feeding on a general basis, but clinical experience suggests that the threshold for trying it should be low.
Parenteral nutrition
Even when enteral feeding is optimised and hypocaloric nutrition in the early acute phase is considered acceptable, enteral feeding is rarely sufficient to achieve desired nutritional targets. Studies have shown that only about 50 % of calculated energy needs can be met with enteral feeding alone (3, 5). Supplementary parenteral nutrition will therefore be required, but there are marked discrepancies between the recommendations in terms of when this should be started.
Previous European guidelines (4, 8) recommended starting parenteral nutrition in the early acute phase, and within 48 hours after admission to intensive care if the patient was unlikely to have their full caloric needs met within 72 hours. This has differed from the American guidelines, in which initiation of parenteral nutrition is not recommended until the late phase, on the eighth day in intensive care at the earliest (5), except in those patients who are severely undernourished. This means that hypocaloric nutrition is accepted and preferred over supplementing with parenteral nutrition. A large randomised controlled study from 2011 compared these two widely differing approaches (early vs late initiation of parenteral nutrition). The patients with late initiation came out of the comparison far better and had fewer infections and complications and shorter stays in intensive care (9).
The study has received some criticism (10), but it has had consequences for the new European guidelines, which have changed and now recommend that parenteral nutrition should not be started in the early acute phase, but rather in the late acute phase between the third and seventh days in intensive care. This is a broad and imprecise recommendation that leaves much scope for clinical evaluation and individual judgement. Given the current evidence base, however, the recommendation is reasonable and reflects the existing knowledge gaps and disagreements in the field.
Nutritional needs and dosing
Both European and American guidelines recommend hypocaloric nutrition for the first 48 hours, at 70–80 % of calculated caloric needs, in order to avoid overnutrition. From day three, the European guidelines recommend a more aggressive approach in which nutritional support can be increased to as much as 100 % of the patient’s requirements and supplementary parenteral nutrition can be provided if necessary. The American guidelines differ radically here: parenteral nutrition is not recommended if enteral feeding provides > 60 % of recommended calorie intake, also in the late phase. Recommended standardised targets are typically 25–30 kcal/kg/day. The problem here is individual variation: the caloric needs of individuals within a patient population have been shown to deviate by an average of 50 % from the standardised target values (10). Individual caloric needs can be estimated via simultaneous measurement of O2 consumption and CO2 production, so-called indirect calorimetry. This can be performed non-invasively, for example by connecting measuring instruments to the ventilator circuit. Individual calculation of caloric needs by means of indirect calorimetry has been shown to reduce complications and improve administration of parenteral nutrition (11). Both European and American guidelines recommend the use of indirect calorimetry. This quantification method should be used to a much greater extent in intensive care patients.
Negative effects of parenteral nutrition
Given the negative clinical consequences of undernutrition described earlier, it might seem paradoxical that late initiation of parenteral nutrition is advantageous compared to early initiation. The reason is that parenteral nutrition has a number of negative effects that have now become more apparent and that have led to its more restricted use. Parenteral nutrition has been shown to increase the risk of infection, multiorgan failure and hyperglycaemia, and to increase the frequency of electrolyte disturbances in the form of refeeding syndrome. During medical nutrition, one should always check for electrolyte imbalances by measuring plasma phosphate, levels of which should not fall below 0.65 mmol/l (3). There has been little research into the causes of these deleterious effects. Singer et al. have shown that the negative effects can be avoided through personalised dosing of nutritional support via indirect calorimetry, and attribute the negative clinical outcomes to overnutrition (11).
Use of glucose and insulin
The guidelines previously recommended a minimum of 2 g/kg/day glucose, but this lower limit has now been removed owing to a lack of evidence. The clinical consensus is still that some intravenous glucose can be administered, but a maximum of 5 mg/kg/min. Use of insulin has been and remains a contentious issue, both during fasting and during ongoing nutritional support in intensive care patients. Hyperglycaemia was generally accepted, until a randomised controlled single-centre study in 2001 showed that intensive insulin therapy and strict normoglycaemia resulted in lower morbidity and mortality (12). A glucose level below 10 mmol/l is now recommended, but hypoglycaemia must be avoided at all costs. For all practical purposes, plasma glucose should be titrated to 6–8 mmol/l, also during parenteral nutrition. However, even with a strong focus on glycaemic control, achieving this is challenging in patients who are receiving parenteral nutrition, and experience suggests that the desired glycaemic control is achieved in only 50 % of patients (13). Medical interventions and algorithms that permit better glycaemic control are therefore likely to improve outcomes for intensive care patients in the future.
