There will always be risks associated with transfusion, including the development of blood group antibodies. This case report emphasises the importance of considering whether transfusion is truly necessary.
A young woman was diagnosed with iron deficiency anaemia with haemoglobin 7.0 g/dl (11.7–15.3 g/dl), probably due to gluten intolerance. She was otherwise in good health. She was blood group B RhD positive with a negative antibody screen. Because of her anaemia, she was transfused with one unit of red cell concentrate.
Prior to blood transfusion, a blood sample from the patient is typed for ABO and RhD (1). In addition, an antibody screen is performed to determine whether the patient has clinically significant antibodies against blood group antigens beyond the ABO system, which is just one of 36 blood group systems with 322 blood group antigens (2). If the antibody screen is negative, the blood bank will issue ABO and RhD compatible red cell concentrate. In addition, women of reproductive age always receive K negative erythrocytes.
Sometime later, the woman became pregnant. At gestational week 14, anti-Ku alloantibody was detected by a routine antibody screen.
Anti-Ku is an alloantibody directed against all antigens in the Kell blood group system. The prevalence of the Ku antigen is approximately 100 % in all populations (3). This means that there are very few individuals who have none of the Kell antigens on their red cells. Individuals with this phenotype, which is called K0, can develop anti-Ku antibodies following transfusion with Ku positive blood or pregnancy with a Ku positive fetus. A severe acute haemolytic transfusion reaction can occur if a patient with anti-Ku antibodies receives a transfusion of Ku positive red cells (3). All red cell concentrates in blood banks in Norwegian hospitals, including concentrates used in case of emergency before a patient is typed for ABO and RhD, will be Ku-positive. Anti-Ku can lead to fetal anaemia, fetal death and haemolytic disease of the newborn (4, 5).
The woman was monitored by specialists in fetal medicine with regular ultrasound examinations, including measurement of blood velocity in the fetal middle cerebral artery to detect early signs of fetal anaemia. The father was O RhD negative, K negative and k positive, thus it was highly likely that the fetal red cells expressed the Ku antigen. Titration of anti-Ku in maternal blood samples revealed an increase in titre from 1:128 to 1:1 024 over the course of the pregnancy. However, repeated measurements of blood velocity in the fetal middle cerebral artery were normal until gestational week 36, with values below 1.5 MoM (multiples of the median) (Figure 1). There was therefore no indication for intrauterine transfusion.
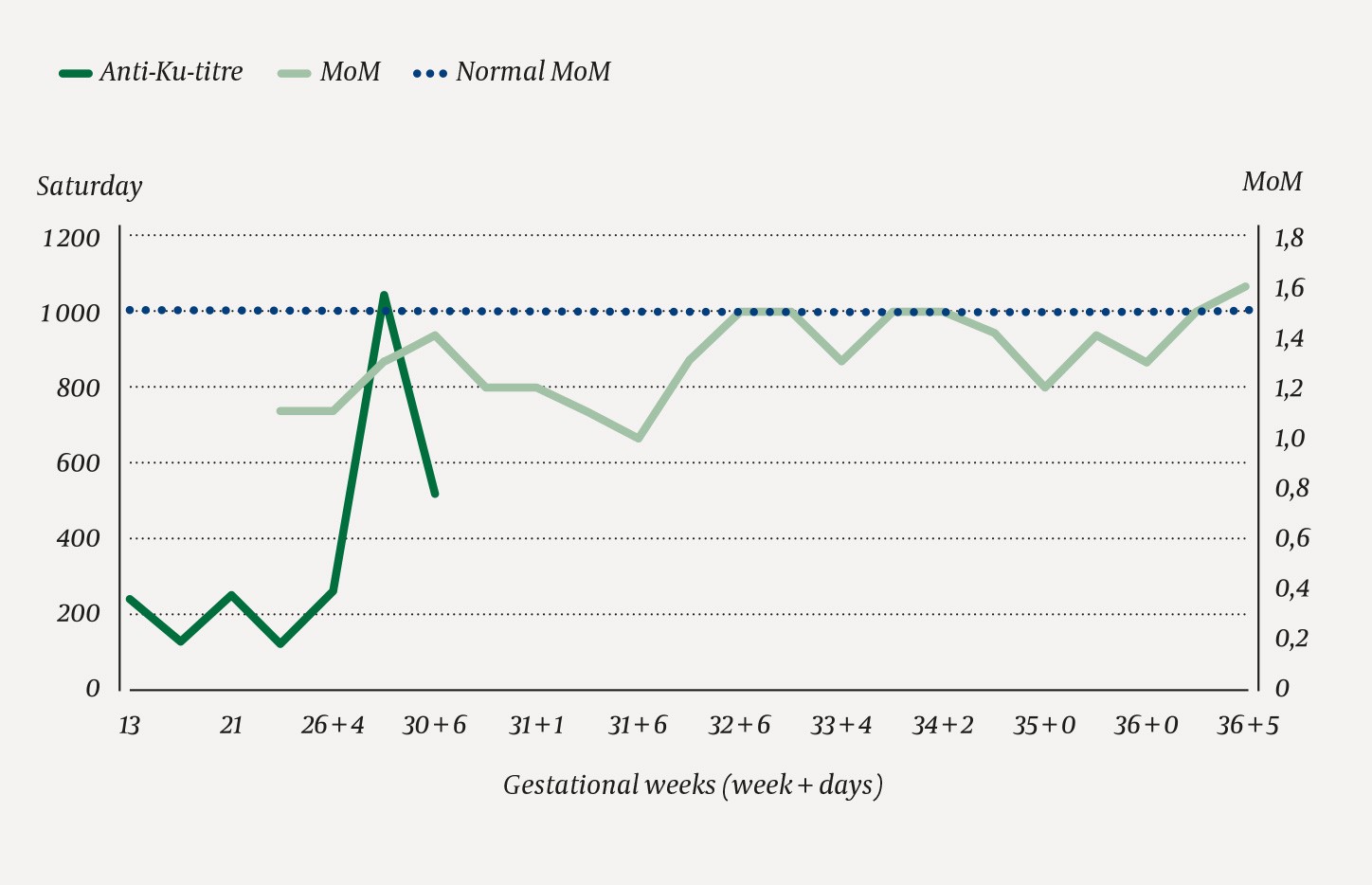
Figure 1 Anti-Ku titre (last titration was performed at gestational week 30) in the pregnant woman and blood velocity (multiples of the median (MoM)) measured in the fetal middle cerebral artery via ultrasound.
An increase in the titre of an antibody indicates increasing antibody concentration. This increase may correlate with the severity of fetal anaemia (6). Between gestational weeks 18 and 34, severe anaemia can be treated with intrauterine transfusions of red cell concentrates that are antigen-negative for the antibodies concerned and compatible with maternal plasma. After week 34, it is preferable to induce delivery.
It was possible the newborn would need blood transfusion. Although the mother had no known risk of haemorrhage, we also wanted to have blood available for the delivery. However, the blood bank had no K0 donors, and only a few frozen K0 red cell concentrates that had been purchased previously. The Norwegian National Advisory Unit on Immunohematology at the Department of Immunology and Transfusion Medicine, Oslo University Hospital, is the only blood bank in Norway with stores of rare frozen units.
Since K0 is very rare worldwide, it can be difficult to obtain compatible blood for K0 patients with anti-Ku antibodies. A search revealed 25 active blood donors in Japan and a few blood banks in Europe with a small number of frozen units. Obtaining fresh units from abroad was difficult because it was not known when the woman would go into labour. In patients with rare blood group antibodies, it may be indicated to collect blood from the patient for autologous transfusion. A red cell concentrate collected in this way has a shelf life of 35 days, the same as for standard red cell concentrates. The unit can also be frozen within a week of collection for later use. The use of autologous blood avoids further immunisation of the patient against other blood group antigens, which would make it even more challenging to find compatible blood in the future. The requirements for autologous donation are less stringent than for normal blood donation; for example haemoglobin levels should normally be at least 12.5 g/dl in female blood donors, but 11 g/dl is an acceptable minimum for autologous donation.
The woman was given oral and intravenous iron therapy owing to her low haemoglobin levels (11.5 g/dl). Blood was collected at gestational weeks 34 and 36, with the latter collection three days prior to induction of labour.
Both autologous red cell units were primarily intended for the mother and were stored at 4 °C. The second unit was to be frozen if not used. The frozen allogeneic K0 red cell concentrates could, if needed, be used for either mother or child, assuming ABO compatibility with the child. The newborn would be ABO typed first after the birth.
The thawing of frozen units takes 6–8 hours, thus they would not be available in case of urgent need for blood transfusion. A thawed red cell concentrate has a shelf life of up to 72 hours and cannot be refrozen. If the newborn required a blood transfusion because of life-threatening anaemia and the frozen units were not thawed, red cell concentrates collected from the mother could be considered (after irradiation and washing) as an emergency solution, provided there was ABO compatibility between mother and newborn. However, transfusion from relatives (so-called directed donation) is not common practice in Norway as the risk of HLA immunisation and infection transmission is greater with this type of transfusion.
At gestational week 36, increasing blood velocity was detected in the fetal middle cerebral artery (maximum systolic velocity equivalent to 1.6 MoM), indicating increasing anaemia. Labour was therefore induced and proceeded without complications or severe maternal haemorrhage. The infant had a birth weight of 2 858 g, which was normal for the gestational age. Umbilical cord blood samples showed haemoglobin 7.5 g/dl (14.5–23 g/dl) and direct antiglobulin test (DAT) 4+. Upon admission to the neonatal intensive care unit, haemoglobin was 11.3 g/dl (14.5–23 g/dl), haematocrit 0.27 (0.42–0.75), and bilirubin 79 μmol/l (<100 μmol/l). The child was typed as O RhD positive, thus there was ABO incompatibility between mother and child. Various blood group O red cell concentrates from the blood bank stock were tested for compatibility with the newborn, but crossmatches were strongly positive. It was then ascertained that the newborn had anti-Ku haemolytic disease.
The strongly positive direct antiglobulin test meant that the newborn’s red cells had bound the mother’s alloantibody, and the strong positive crossmatches were due to free anti-Ku that had crossed the placenta. Bilirubin levels must be closely monitored in cases of postnatal haemolytic disease, as there is a risk of brain damage due to hyperbilirubinaemia. Mild hyperbilirubinaemia/haemolytic disease can be treated with phototherapy. In more severe cases, treatment is supplemented with high-dose intravenous immunoglobulin (7, 8). If the treatment does not prove sufficiently effective, an exchange transfusion may be indicated in which bilirubin, antibody-bound red cells and free maternal antibody are removed from the circulation of the newborn (9). These are replaced with red cells that are antigen-negative for the maternal antibody, compatible with the maternal plasma, and plasma compatible with the ABO type of the newborn. Maternally derived antibodies will eventually break down, but in severe cases anaemia may persist for up to several weeks.
Treatment with intravenous immunoglobulin (0.5 g/kg) and phototherapy were started on postnatal day 1 (Figure 2). On postnatal day 2, the child’s haemoglobin level fell to 8.4 g/dl. The child was clinically unaffected, and had high reticulocyte counts with a peak of 500 · 109/l (30–100 · 109/l) on day 5. The highest bilirubin level (232 μmol/l), which was measured on postnatal day 6, was below the phototherapy threshold. The blood bank had only a single compatible frozen blood group O K0-red cell concentrate, which was not enough for an exchange transfusion. This unit was saved in case an emergency transfusion should ever be needed, and intravenous immunoglobulin therapy (1 g/kg) was continued. The newborn was discharged at the age of one week with a haemoglobin level of 9.4 g/dl. A follow-up appointment two days later showed increased haemoglobin of 10.1 g/dl. At three weeks of age, the child was readmitted with haemoglobin of 7.9 g/dl (age 1–4 weeks: 12.5–21 g/dl) and rapid respiration rate. Another dose of intravenous immunoglobulin (1 g/kg) was administered. Thereafter, the haemoglobin level gradually increased to 10.9 g/dl (age 2–5 months: 9–14 g/dl) at three months of age. The child was breastfed, received iron supplementation and showed good weight gain and normal development.
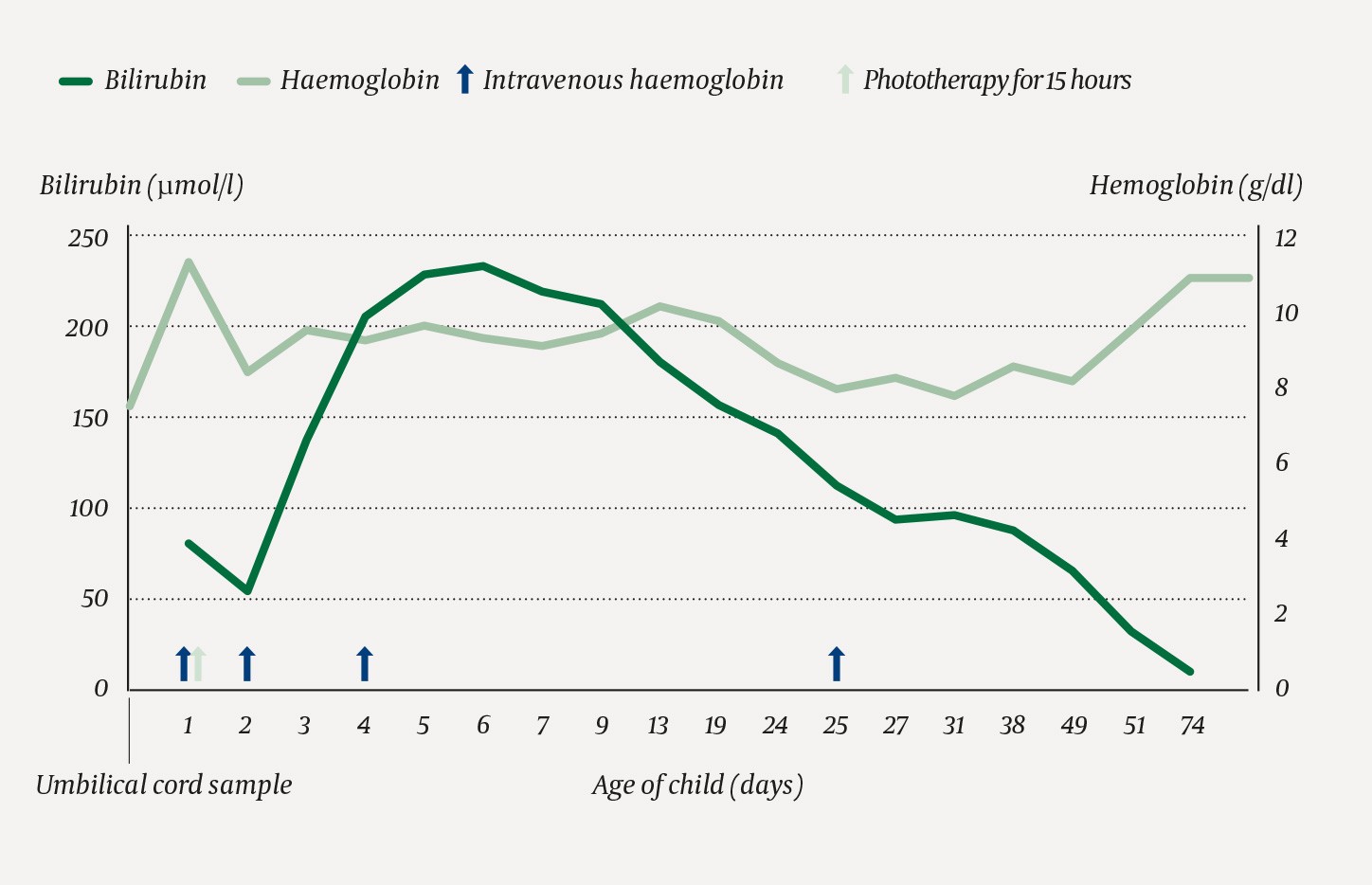
Figure 2 Bilirubin and haemoglobin levels in the neonate. Treatment with intravenous immunoglobulin (first two doses: 0.5 g/kg, then 1 g/kg) and phototherapy are indicated by arrows.
The last collected autologous unit was frozen down. The woman has been encouraged to continue with autologous blood donations once she has finished breastfeeding, to build up a stock of autologous, frozen red cell concentrates to meet any future transfusion requirements.
Discussion
Individuals who are exposed to foreign blood group antigens they do not express themselves, either through transfusion or pregnancy, can form antibodies that may cause complications upon subsequent transfusion with antigen-positive blood or pregnancy with an antigen-positive fetus (Figure 3). We report the case of a complicated pregnancy in which a transfusion resulted in immunisation. The haemoglobin level measured in the umbilical cord sample was lower than that measured in the neonatal intensive care unit (7.6 g/dl versus 11.3 g/dl). The reason for this difference is unclear, and we cannot rule out the possibility that the high value was due to measurement error as the haemoglobin was 8.4 g/dl the following day.
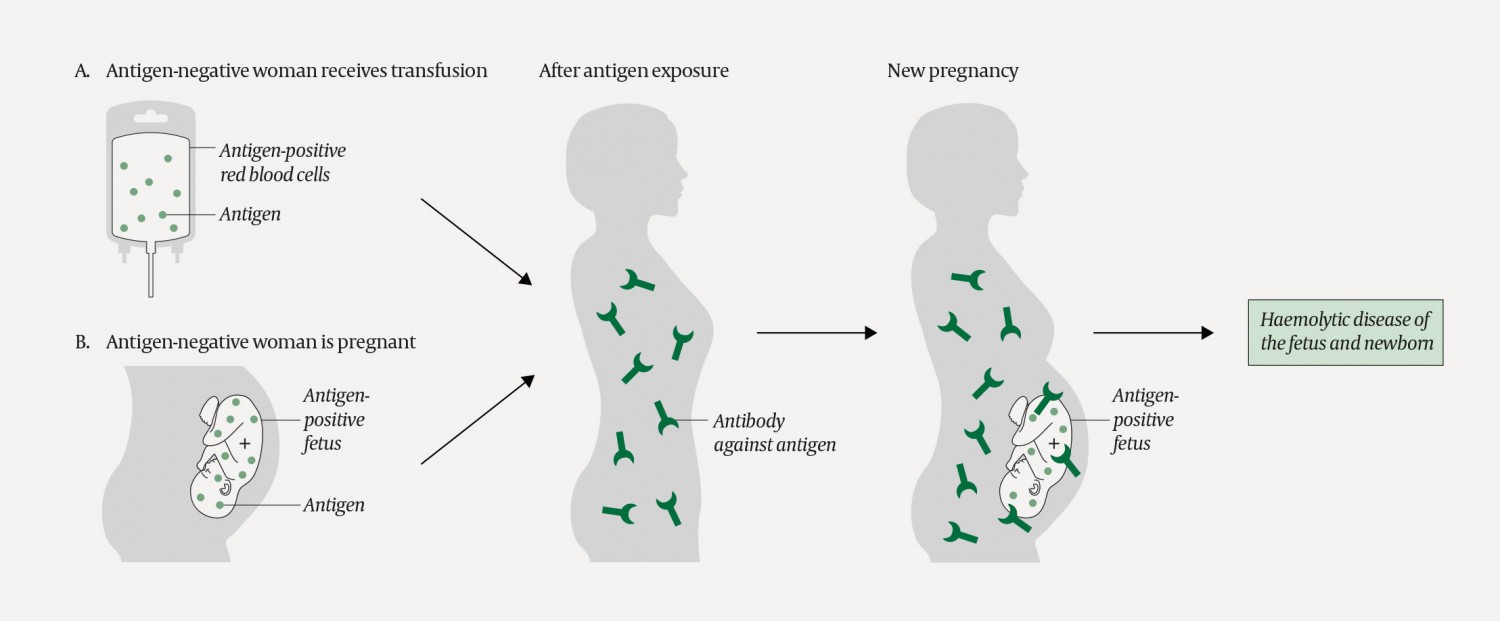
Figure 3 A woman who is exposed to (an) antigen(s) she does not express on her own red cells may form blood group antibodies against those antigens. Antigen exposure may occur via a transfusion (as in the current patient) or pregnancy. If the woman later becomes pregnant and the fetus is antigen-positive, the woman’s antibodies can lead to haemolytic disease of the fetus and newborn.
Antibody screening in pregnancy aims to detect blood group antibodies that can lead to haemolytic disease of the fetus and newborn. Anti-D is the most common culprit. Other antibodies such as anti-C, -c, -E and -e in the Rh system can also give rise to the disease, but rarely when antibody titres are lower than 1:128. Antibodies against blood group antigens in the Kell system can, even at low titres, cause severe haemolytic disease of the fetus and newborn, and even fetal death. Kell antigens are also expressed on red cell precursors, with the result that these are destroyed after antibody binding, inhibiting erythropoiesis and causing long-lasting anaemia (10). Other blood group antibodies can also cause disease in rare cases. In haemolytic disease, immune-mediated destruction of fetal red cells leads to anaemia, which in severe cases can result in hydrops fetalis and fetal death. Maternal blood group antibodies of the IgG type cross the placenta and bind paternally inherited antigens on fetal red cells. These red cells are destroyed by macrophages in the liver and spleen, resulting in anaemia and hyperbilirubinemia. During pregnancy, bilirubin is transported across the placenta and conjugated in the mother’s liver. However, the neonatal liver has only a limited capacity to conjugate bilirubin, and excess unconjugated bilirubin can lead to severe brain damage (kernicterus).
Antibody screening is performed in gestational weeks 12–16. In RhD negative women, the fetal RhD type should also be determined in gestational week 24 using non-invasive prenatal testing (NIPT) based on cell-free fetal DNA in the maternal blood (11). If the fetus is RhD positive, the woman is given RhD prophylaxis in gestational week 28. Postpartum RhD prophylaxis is given if the newborn is typed as RhD positive from the umbilical cord sample.
If a blood group antibody that can lead to fetal anaemia is detected, the antibody is titrated. The first follow-up sample is taken in gestational weeks 18–28 depending on which antibody was detected. Further samples are taken at least every four weeks, in consultation with a specialist in obstetrics and gynaecology (1). The fetus is monitored through measurement of blood velocity in the middle cerebral artery at a centre for fetal medicine. The Norwegian National Centre for Fetal Medicine at St. Olavs University Hospital, Trondheim has responsibility for intrauterine transfusions. About five pregnant women receive intrauterine transfusions each year.
The introduction of postpartum RhD prophylaxis in 1969 led to a clear reduction in the incidence of haemolytic disease of the fetus and newborn (12). A further reduction is anticipated as a result of the nationwide introduction in 2016 of RhD prophylaxis in gestational week 28 for RhD negative women carrying an RhD positive fetus (11). Nevertheless, there is still a risk of immunisation in cases of fetomaternal haemorrhage prior to gestational week 28, and in women from countries without an optimal programme for prenatal and postpartum prophylaxis. Prophylaxis is given to prevent immunisation in the mother. If the mother is already immunised and has formed anti-D antibodies, prophylaxis will have no effect. There is no prophylaxis available for the other antibodies. It is therefore important to prevent antibody formation in girls and in women of reproductive age. RhD negative individuals aged <50 years should be transfused with RhD and K negative red cells. When antibody screening prior to a transfusion is negative, other antigens are not taken into account, and therefore any such antigens in the transfused blood will have the potential to immunise.
Transfusions in Norway are generally safe, but the risk of complications can never be completely eliminated. Transfusion should only be performed after careful consideration and when there is an appropriate indication. Alternatives to transfusion should be considered where possible. In 2018, the Norwegian Medical Association launched a campaign based on similar initiatives in the United States, Canada and Australia – ‘Choosing wisely’ [Gjør kloke valg] – in which each medical specialty is to develop recommendations to improve patient care and safety. One of the recommendations for transfusion is as follows: Avoid blood transfusion as a treatment for iron deficiency anaemia (13). This case report illustrates how transfusion of one single unit of red cell concentrate can lead to the formation of a clinically significant blood group antibody. In this patient with non-life-threatening anaemia, iron therapy would probably have achieved the desired outcome without need for transfusion. This would have avoided the immunisation that led to a complicated pregnancy. Future pregnancies will entail a risk of haemolytic disease of the fetus and newborn at least as severe as in the current case. When there are no antigen-negative blood donors, managing any transfusion requirements in the mother or child will be highly challenging.