A man in his 70s who was being treated for Waldenström’s macroglobulinaemia developed progressive pain in his lower extremities and symptoms consistent with sepsis. Neurological symptoms arose during a break in treatment. Extensive workup finally revealed a rare underlying cause.
A man in his 70s with known epilepsy for many years had been diagnosed with Waldenström’s macroglobulinaemia some years previously. The disease had initially been observed without treatment, but after two years treatment had commenced with bendamustin (alkylating chemotherapy) and rituximab (monoclonal anti-CD20 antibody) because of increasing anaemia. He had responded well, but slowly. Twenty months later he had again developed increasing anaemia, and the treatment chosen was ibrutinib (a Bruton’s tyrosine kinase inhibitor), to which he responded very well after a short time.
Waldenström’s macroglobulinaemia is an indolent non-Hodgkin’s B cell lymphoma that affects the bone marrow and sometimes the spleen and lymph nodes. The disease is characterised by monoclonal immunoglobulin M (IgM) in serum. The most common manifestation is anaemia as a consequence of bone marrow failure. There may also be other cytopaenias, general symptoms (‘B’ symptoms), symptoms due to splenomegaly or lymphadenopathy, hyperviscosity symptoms due to high IgM concentration or peripheral neuropathy (1, 2).
The patient contacted the haematology clinic between two check-ups. Three months after starting ibrutinib he developed slight oedema and pain in his feet – first on the dorsal left foot, then also the dorsal right foot, then spreading to ankles, calves and knees. He experienced pain on moving his joints, both with and without loading, but was free of pain when at rest.
Joint pain is a common adverse effect of using ibrutinib. The manufacturers were therefore contacted, and could not exclude the possibility of an adverse effect. They proposed treatment with paracetamol and celecoxib. These were tried, but the effect was limited. The daily dose of ibrutinib was reduced from 420 mg to 280 mg in accordance with the recommendations in the manufacturer’s SPC. However the symptoms worsened, and he developed a pronounced limp. In consequence, the drug was temporarily discontinued. The SPC states that approximately 6 % of patients on ibrutinib reduce the dose due to adverse effects, and that 5 % discontinue therapy (3).
Three days after discontinuing ibrutinib, the patient contacted the clinic again, as his general condition suddenly worsened severely and the pain in his right leg increased. His walking was now very poor, he had tremors that were interpreted as possible chills, and was clinically subfebrile. There were crackles over both lung bases and pneumonia was suspected. The patient was hospitalised immediately.
A clinical examination of the lower extremities did not reveal erythema, warmth, oedema or tenderness to palpation. Findings for other clinical tests were also normal apart from crackles over the lungs. Vital parameters were stable with blood pressure 129/54 mm Hg, pulse 96 beats/min. and respiration frequency 20 per minute. Body temperature was 38.8oC and oxygen saturation 95 % without supplemental oxygen. Blood tests showed SR 70 mm/hour (reference range 1–28 mm/hour), haemoglobin (Hb) 11.3 g/dl (13.7–16.5 g/dl), leukocytes 8.6 ∙ 109/l (3.9–9.5 ∙ 109/l), neutrophil granulocytes 5.6 ∙ 109/l (1.5–5.7 109/l), C-reactive protein (CRP) 265 mg/l (0–4 mg/l), procalcitonin 0.2 µg/l (< 0.1 µg/l), fibrinogen 6.3 g/l (2.0–2.4 g/l), D-dimer 2.0 mg/l (0.2–0.4 mg/l) and IgM 3.21 g/l (0.40–2.10 g/l). Chest x-ray revealed possible infection-related bilateral opacities basally. Shortly after admission, therapy with intravenous penicillin and gentamicin was started on suspicion of incipient sepsis originating in the airways.
Airway infections and sepsis are also known adverse effects of ibrutinib (3). However the aetiology of the lower limb pain remained uncertain. Active cancer implies an increased risk of deep vein thrombosis, but the patient had a low Wells score (probability assessment of deep vein thrombosis) and no clinical signs of venous thrombosis other than pain in the lower extremities (4). Infectious or rheumatic disease with septic arthritis was considered as a possible differential diagnosis, but the absence of swelling, warmth and redness weighed against it. Osteomyelitis was another differential diagnosis that might have explained the lower limb pain on loading, fever and rise in CRP, but was excluded by an MRI scan. A fracture was also considered because of the loading-related pain, but was regarded as unlikely because of the absence of trauma in the clinical history and the migratory tendency, bilateral pain and fever in the same period.
We did not suspect progression of the lymphoma, as the patient had a stable, low IgM, which in previous months had correlated well with other parameters indicative of low disease activity, for example Hb. We continued to suspend ibrutinib due to suspicion of a drug-related adverse effect or interaction. However, the clinical pharmacist found no grounds for relevant interactions following an interaction analysis.
During the course, the patient was clinically stable and generally relatively well. Microbiological cultures were negative. He developed no symptoms of focal infection and had fluctuating fever and CRP curves despite broad-spectrum antibiotics therapy. We considered it unlikely that he had an infection requiring antibiotics, and terminated the antibiotics. The patient continued to have a fluctuating temperature peaking at up to 39oC and CRP values of up to 200. The pain was most pronounced in his right lower limb, so that he was unable to place his full weight on it. All autoimmune rheumatology tests were negative. A new examination revealed that the patient had normal passive and active joint mobility, and no pain over the joint spaces or on testing the cruciate ligament and meniscus. X-rays of the lower extremities revealed no radiological evidence of skeletal damage or arthrosis, and only a slight reduction of the medial knee joint spaces bilaterally.
He was formerly a fit walker, but a week after hospitalisation the patient was in such pain when he walked and so unsteady that he had to use a walker to get to the toilet and back. In a new, detailed clinical history, the patient mentioned that for the past six months he had experienced paraesthesia in his lower limbs. The information about paraesthesia raised suspicion of a neurological condition, and a neurological examination revealed ataxia, considerable intentional tremor, left-side dysdiadochokinesia and reduced superficial sensibility in both lower extremities distal to the knees. The patient had good isometric strength, but could not do either toe or heel walking. Because of these findings, we performed a lumbar puncture and ordered an MRI of the cerebrum and medulla. We conferred with the neurologists, who recommended supplementary neurography of the upper and lower extremities.
At this point, because of the patient’s symptoms, added to unclear muscular and skeletal system findings, we considered a paraneoplastic condition to be a possible differential diagnosis. The transformation of Waldenström’s macroglobulinaemia into diffuse large B-cell lymphoma was also considered, but this is relatively rare and improbable with low lactate dehydrogenase and IgM at the onset of symptoms (5).
The patient also had a CT scan of the neck, thorax, abdomen and pelvis, but no evidence was found of a new primary tumour. On the other hand, a definite increase was found in known lymph node tumours in the abdomen, axillae and groin. Biopsies were taken of the abdominal lymph node conglomerate and of bone marrow.
With the neurological symptoms in mind, an MRI scan of the cerebrum was performed on the same day as the neurological examination. This showed three subcortical hyperintensities of uncertain significance. An MRI of the lumbosacral column was described as having no medullary pathology and adequate space in the spinal canal and around the intervertebral nerve roots, but with diffuse bone marrow infiltration. The cerebrospinal fluid was colourless and clear, but with slightly elevated protein and leukocyte values of 1.05 g/l (0.20–0.40 g/l) and 10 ∙ 109/l (0–4 ∙ 109/l), respectively. PCR tests for neurotropic virus and a bacteriological culture of the cerebrospinal fluid were negative. Flow cytometric immunophenotyping of the cerebrospinal fluid was not ready at this time.
Neurography of the peroneal, tibial, sural, median and ulnar nerves revealed considerable sensory polyneuropathy in the lower extremities and slight to moderate motor affection with an axonal injury pattern.
In light of the patient’s known lymphoma, the cerebrospinal fluid findings and changes in the MRI cerebrum raised suspicion that the lymphoma had affected the central nervous system. We could not say definitely whether the polyneuropathy was related to the lymphoma or the treatment, or whether it had other causes.
Seven days after admission, and two weeks after the discontinuation of ibrutinib, his IgM concentration was checked. It had then risen to 17.2 g/l, which indicated progression of Waldenström’s macroglobulinaemia. The patient had also developed anaemia again, with Hb 9.1 g/dl.
Treatment with ibrutinib (420 mg by mouth) was restarted, as the lower extremity pain had not improved after the discontinuation, and an adverse drug reaction was thus less probable. The patient benefited from the treatment, with normalisation of Hb and IgM and regression of the neurological symptoms.
The lymph node and bone marrow biopsies were consistent with Waldenström’s macroglobulinaemia. No evidence was found of transformation into a high-grade lymphoma. At this point we received the results of flow cytometric immunophenotyping of the cerebrospinal fluid, which showed findings of monoclonal B-cells, consistent with lymphoma cells.
The patient’s lymphoplasmacytic lymphoma and neurological symptoms and the findings of lymphoma cells in the cerebrospinal fluid indicated central nervous affection by the known lymphoma, a condition described in the literature as Bing-Neel syndrome.
After the diagnosis Bing-Neel syndrome had been made, we studied the MRI images of the cerebrum and lumbosacral column again and found pathologically thickened and contrast-enhanced meninges over both cerebral hemispheres (Fig. 1) and the cerebellum, and wavy, thickened cauda equina roots (Fig. 2). We concluded that the thickened meninges were an expression of diffuse affection of the central nervous system, consistent with Bing-Neel syndrome. The finding of thickened cauda equina roots was also assumed to be related to the syndrome, but this could not be determined with certainty since the changes were limited, and the images had unfortunately been taken without contrast.
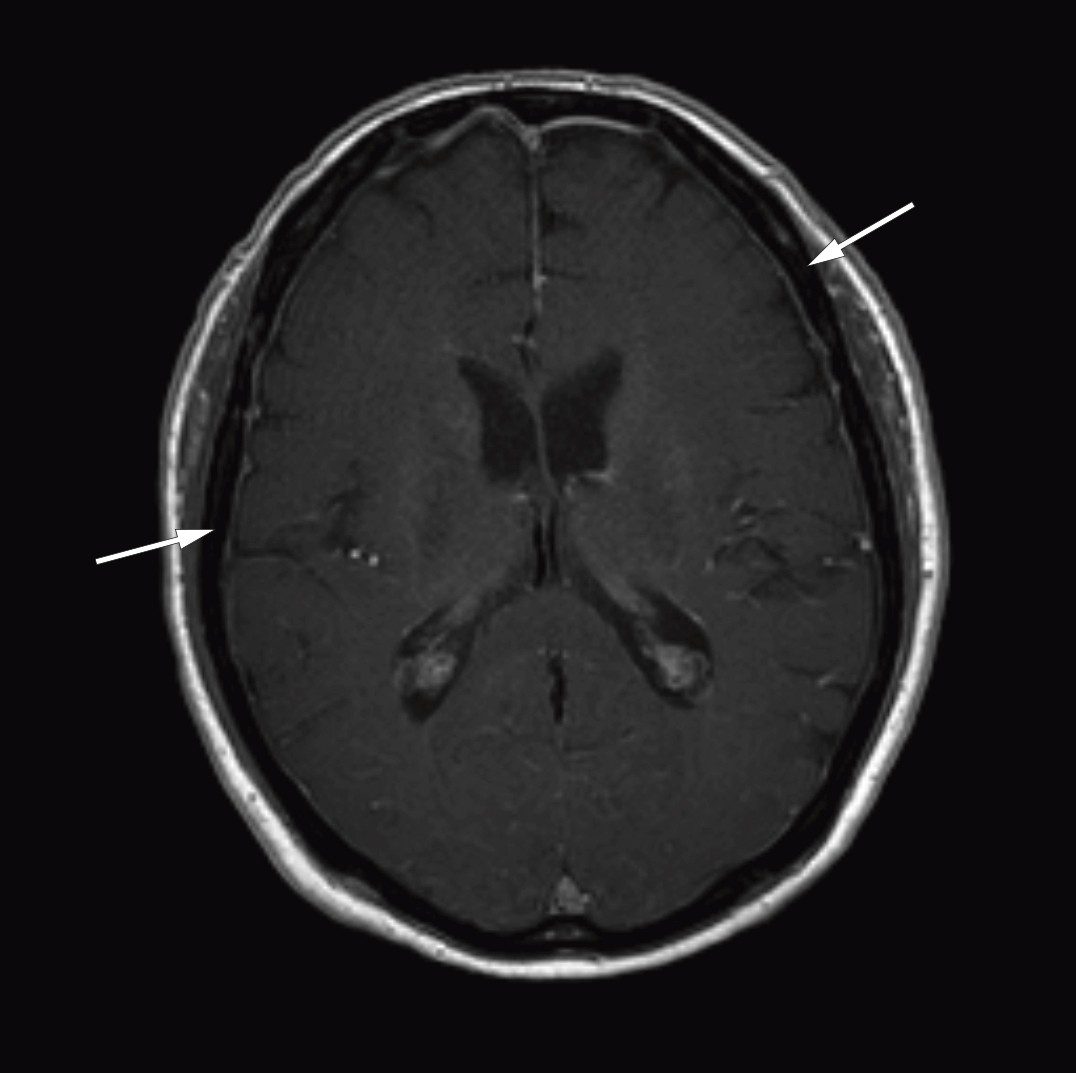
Figure 1 Cerebral MRI after injection of contrast medium (axial section) shows thickened and contrast-enhanced meninges over both cerebral hemispheres.
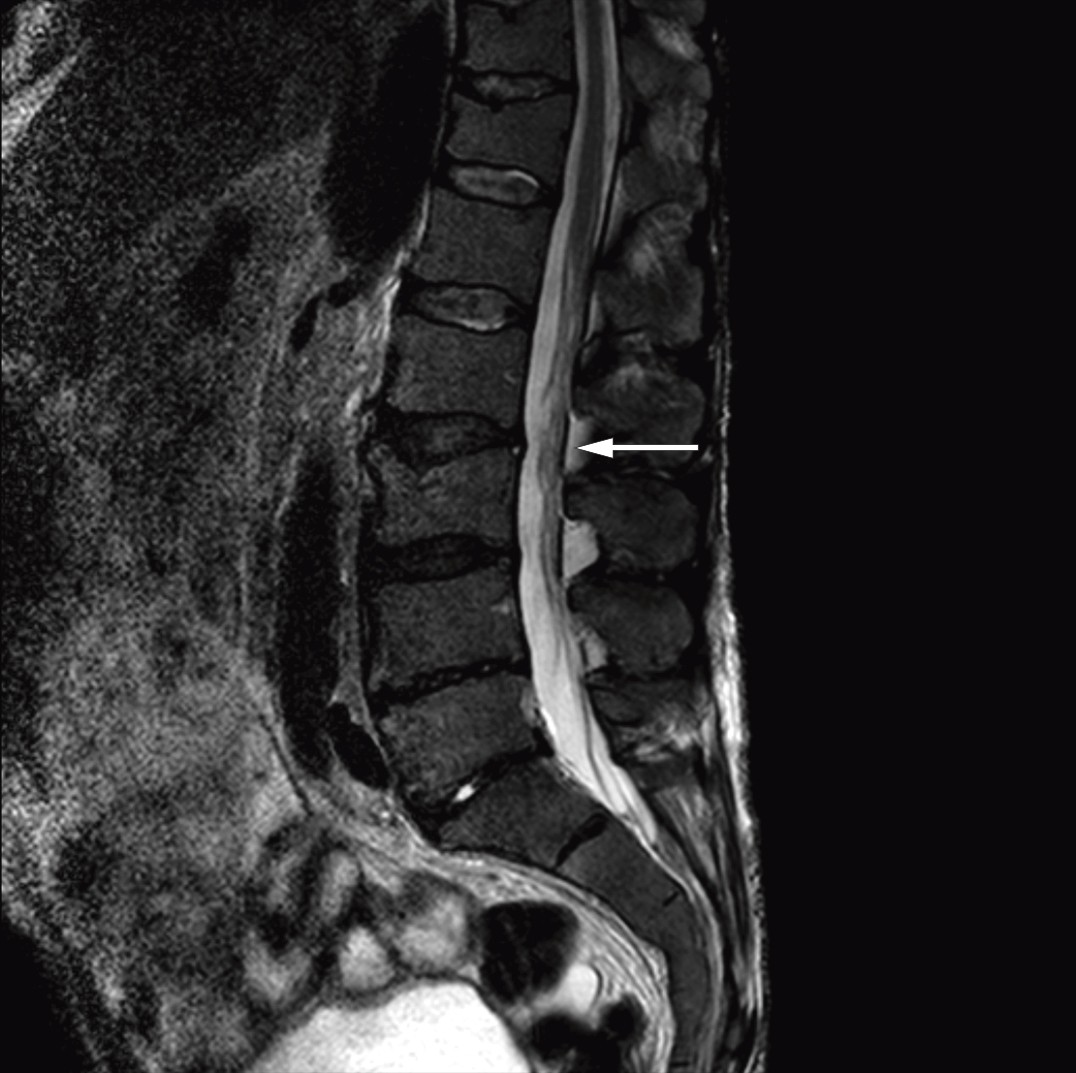
Figure 2 Sagittal T2-weighted MRI image of the lumbosacral column shows wavy, thickened cauda equina roots.
The clinical picture was complex, with three different issues. The first, the lower limb pain, has subsequently proved to be due to subchondral fractures of the femoral condyles and patella, and the patient is now being monitored by an orthopaedic specialist. One possible explanation for the pain is that the lymphoma in the bone marrow has broken down parts of the bone structure, but through its presence also replaced some of the lost support function that the loss of bone represents. The rapid regression of the lymphoma on treatment may have caused a weakening of the support function of the bone marrow until the bone tissue is built up again. This temporary loss of volume may have been the cause of the patient’s fractures.
In addition the patient had fever for a period, which in retrospect we suspect may have been secondary to the lymphoma flaring up.
The third issue was the neurological symptoms, which were probably a direct consequence of the disease process affecting the cerebral parenchyma, the meninges including the root sheaths around cranial nerves and lumbar nerves, with affection of motor and sensory pathways.
The patient subsequently described distress due to increasing tremor, possibly consistent with ataxia, which had begun several months before his actual hospitalisation. This problem had regressed during treatment with ibrutinib, but rapidly recurred after the temporary discontinuation. This indicates that ibrutinib had an effect on a probable central nervous manifestation of a lymphoma that had been present for a long time.
We chose to reintroduce the ibrutinib therapy, but at a higher dose of 560 mg by mouth daily, as described in several studies and retrospective case reports on Bing-Neel syndrome (6).
Discussion
Waldenström’s macroglobulinaemia is a low-grade lymphoproliferative disease that may have a rare manifestation: affection of the central nervous system, also called Bing-Neel syndrome. The syndrome was first described by the Dutch doctors Bing and Neel in 1936, i.e. eight years before Waldenström’s macroglobulinaemia was described.
There is limited literature on Bing-Neel syndrome and it is based mainly on retrospective case reports. In consequence, there is no consensus on diagnostic criteria, treatment guidelines or exact incidence figures. Incidence, as indicated by retrospective studies, is moreover probably underestimated because of deficient knowledge of the condition. The disease has not previously been described in the Norwegian literature.
Bing-Neel syndrome may arise in patients with known Waldenströms macroglobulinaemia, even in the absence of systemic progression, and in previously undiagnosed patients. The average age at the time of diagnosis is 62.4 years (7). In a French study, Bing-Neel syndrome was the first manifestation of Waldenström’s macroglobulinaemia in 36 % of cases (8). The symptoms of Bing-Neel syndrome that present are very heterogeneous, and may include balance and gait disorders, cranial nerve palsy, sensory defects, cauda equina syndrome, neck and/or back pain, headache, visual disturbances, cognitive impairment and pareses (8).
Bing-Neel syndrome is diagnosed on the basis of findings from neuroradiology, cerebrospinal fluid examination and histopathology. Cerebrospinal fluid analysis should not form a routine part of a diagnostic workup of Waldenström’s macroglobulinaemia, but should be strongly considered when there is clinical suspicion of affection of the central nervous system. Some articles mention a biopsy of the cerebrum or meninges as the gold standard for reaching a diagnosis, but this is more relevant in cases without findings through less invasive methods. In the aforementioned French study, MRI abnormalities occurred in 78 % of cases (8). A distinction is made between a diffuse and a tumoral form; the latter is considerably rarer. However, MRI findings alone are not enough; at the same time, the absence of fitting pathological MRI findings does not exclude the syndrome (9).
The treatment options are many, which reflects the fact that the treatment has not been standardised either. First-line treatment may consist of systemic chemotherapy that penetrates the blood-brain barrier, monoclonal antibodies, intrathecal chemotherapy or radiation (10). Ibrutinib is a fairly recent treatment option that is being investigated in several ongoing studies. It has proved to penetrate the central nervous system well and has produced promising results (6, 11).
After first-line therapy, the aforementioned French study found a response rate of 70 % (8). The median time to relapse was 16.5 months, but 70 % responded to second-line therapy. The five- and ten-year survival rates after diagnosis of Bing-Neel syndrome were 71 % and 59 %, respectively (8).
Conclusion
Bing-Neel syndrome is probably an under-diagnosed disease and may precede or follow diagnosis of Waldenström’s macroglobulinaemia. In the sparse existing literature, the disease appears to have a relatively good prognosis with treatment. An awareness of the condition is therefore important. The diagnosis should be considered for patients with known Waldenström’s macroglobulinaemia who present with recent neurological symptoms, but should also be considered by neurologists who are evaluating diffuse neurological symptoms. Bing-Neel syndrome has attracted increasing attention in research circles in recent years. Hopefully this will result in due course in consensus on diagnosis and treatment.