A man in his sixties developed acute back pain with radiation to the left thigh. Healthcare professionals initially suspected venous thrombosis or sciatica. Four hours after pain onset, the patient had paralysis and decreased sensation in both lower limbs. Red flags in the Norwegian clinical guidelines for the management of low back pain do not cover this patient’s diagnosis.
An active man in his early sixties developed acute low back pain with radiation to the medial and anterior left thigh as he stood in the bathroom one morning, brushing his teeth. After an hour, he called the Accident and Emergency (A&E) department. He mentioned that he was taking 5.5 tablets of warfarin (Marevan) weekly owing to previous pulmonary embolism, and that a week previously he had started taking candesartan tablets (8 mg daily) because of hypertension. The nurse in A&E suspected venous thrombosis, and a paramedic was dispatched to examine him. During the examination, the patient developed acute weakness in his left leg. The paramedic found no signs of venous thrombosis and concluded that the most likely cause was sciatica. He advised the patient to rest and see whether the pain resolved. The patient fell asleep, but 90 minutes later he awoke with persistent back pain and weakness, now accompanied by complete loss of sensation in the left leg. He called the Emergency Medical Communications Centre (EMCC) again, but was told to wait as there were no ambulances available. Half an hour later, the patient called the EMCC yet again. By this point he had reduced sensation and increasing weakness in both legs. An ambulance arrived approximately four hours after the onset of pain.
The patient had no previous history of back pain and could not relate the pain to any precipitating event. Both the nurse in A&E and the paramedic who examined the patient suspected the most obvious diagnoses, such as venous thrombosis and sciatica.
In common with international recommendations (1), the Norwegian clinical guidelines for management of low back pain [‘Nasjonale kliniske retningslinjer for korsryggsmerter’] divide low back pain into three categories that form the basis for further action. These are: a) non-specific low back pain, b) nerve root involvement, and c) possible serious underlying spinal pathology or cauda equina syndrome, such as a fracture, tumour or infection. The last category is marked with red flags (2). Red flags are warning signs in the medical history and in clinical findings, and are used to alert clinicians to symptoms and signs that may be significant for the follow-up of individual patients with low back pain. ‘A patient over the age of 55 who develops back pain for the first time’ and ‘constant pain, possibly increasing over time, pain at rest’ are examples of red flags, and might have indicated that our patient required additional follow-up (see Box 1).
Box 1 Warning signs of possible serious underlying pathology (2). Red flags.
Back pain that begins, or that is perceived to differ from previous back pain, below the age of 20 or above the age of 55
Constant pain, possibly increasing over time, pain at rest
General malaise, fever and/or weight loss
Trauma, cancer, use of steroids or immunosuppressants, substance abuse
Widespread and possibly progressive neurological deficits
Spinal deformity
High ESR, pronounced morning stiffness lasting more than one hour
Half an hour after his last contact with the EMCC, the patient arrived at Acute Admissions of a university hospital. The patient’s medical history was reviewed and important information emerged on the reasons for his anticoagulant therapy. Nine years prior to the current event, the patient had suffered bilateral pulmonary embolism and arterial embolism in his upper right arm. The upper limb embolus was removed surgically on account of limb-threatening ischaemia, and he was treated with warfarin for several months. Two years after the surgery, the patient experienced recurrence of the pulmonary embolism and was then placed on lifelong warfarin therapy. Three years prior to the current event, he developed paroxysmal atrial fibrillation and was treated with pulmonary vein isolation and isthmus block. The following year, all four pulmonary veins were isolated following recurrence of the atrial fibrillation. His INR value had been checked regularly and had remained stable within the therapeutic range (2.0–3.0).
Upon arrival at the university hospital this time around, the patient had low back pain with a visual analogue scale (VAS) score of 6 out of 10, and the pain increased upon mobilisation. He was nauseous and had urinary retention and no control of the anal sphincter. Examination of the lower extremities revealed complete paralysis distal to and including the hip joint. He had decreased sensitivity to pinprick and light touch in both thighs, most pronounced in the left thigh, as well as decreased temperature sensation in the entire left lower extremity, and distal to and including the knee in the right lower extremity. He had extinguished joint position sense and bilaterally extinguished patellar and Achilles tendon reflexes. He scored 2–3 on the Modified Neuro-Grade scale (2 = para- or tetraparesis, 3 = para- or tetraplegia) (3).
The length of time from the patient developing acute radiating back pain to his experiencing symptoms of spinal cord injury or compression, with paraparesis, extinguished patellar and Achilles tendon reflexes, urinary retention and flaccid sphincter tone, was 4.5 hours. The patient lived in the vicinity of a university hospital, and after examination in Acute Admissions, he was quickly transferred to the neurosurgical department for examination and treatment.
Examination upon admission showed blood pressure 146/68 mm Hg, heart rate 70 beats/min, Hb 15.4 g/dl (reference range 13.4–17.0 g/dl), platelets 241 ∙ 109/l (145–390 ∙ 109/l), creatinine 72 µmol/l (60–105 µmol/l) and Quick INR 2.6 (within the therapeutic range of 2–3). MRI revealed an intraspinal subdural haematoma at levels Th9–L1 with dislocation of the spinal cord and medullary cone (Figure 1). CT angiography showed no evidence of vascular malformation or arteriovenous fistula. Based on interpretation of an acute MRI, the patient was given phytomenadione (Konakion, 10 mg) and prothrombin complex concentrate (Octaplex, 2 500 IU), dosed according to the recommendations of the Neurology-Norwegian Electronic Medical Handbook (‘NevroNEL’) (4), to avoid further haemorrhaging.
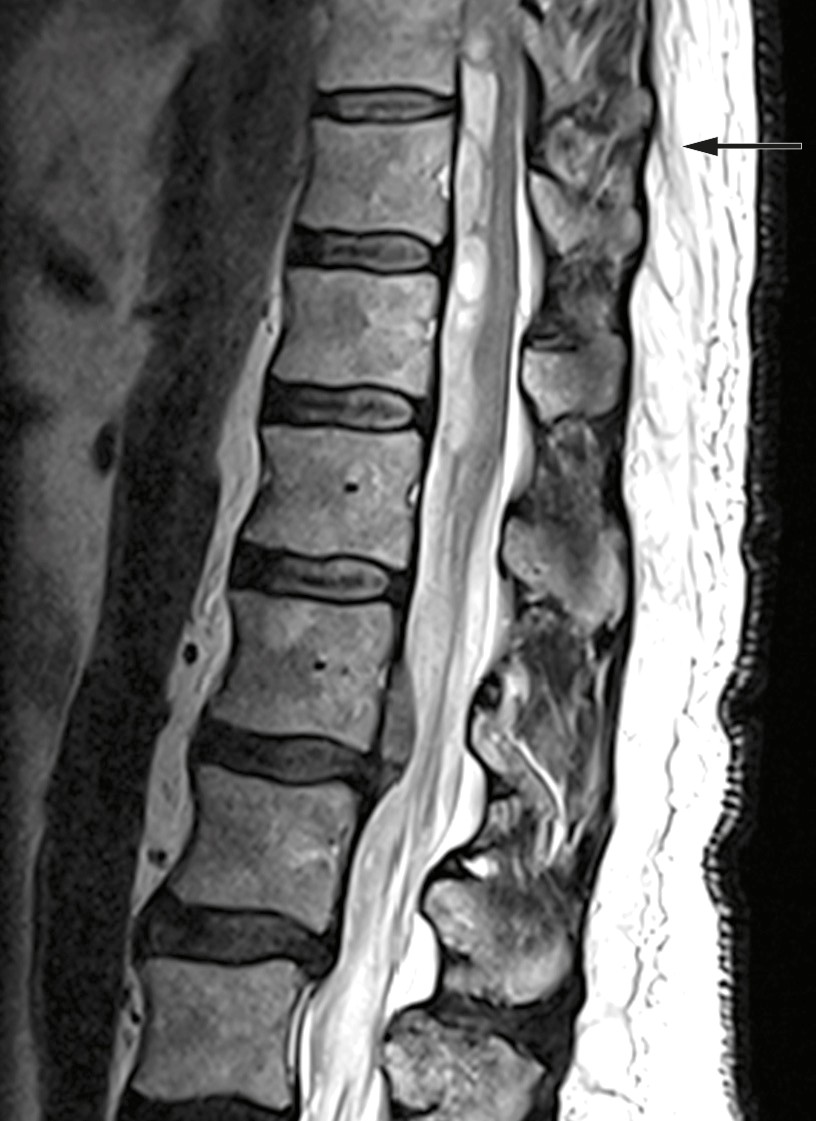
Figure 1 Preoperative MRI: Intraspinal subdural haematoma at levels Th9-L1 and dislocation of the spinal cord and medullary cone.
Anticoagulation therapy is the most frequent cause of spinal cord haemorrhage, particularly for subdural and epidural haematomas, in patients aged 46–75 years (5) (6). Upon admission, the patient’s INR value was 2.6. Warfarin was discontinued and the patient was given an antidote in accordance with standard guidelines.
The patient was signed up for emergency surgery under general anaesthesia. The knee-elbow position was used, along with X-ray imaging, midline incision and three-level laminectomy. No epidural haematoma was seen during the surgery, but the blue discoloured dura mater was opened. The subdural haematoma was gently evacuated, and there was no major source of bleeding or difficulty in achieving haemostasis. The spinal cord showed bluish discolouration, and significant subarachnoid and intramedullary haemorrhage was suspected. Closure was then performed under good haemostasis. Postoperative MRI showed a reduced subdural haematoma at laminectomised levels Th9–11, unchanged subdural haematoma at level Th12, new intramedullary signal changes at Th10–L1, and subarachnoid blood caudal to Th12 (Figure 2).
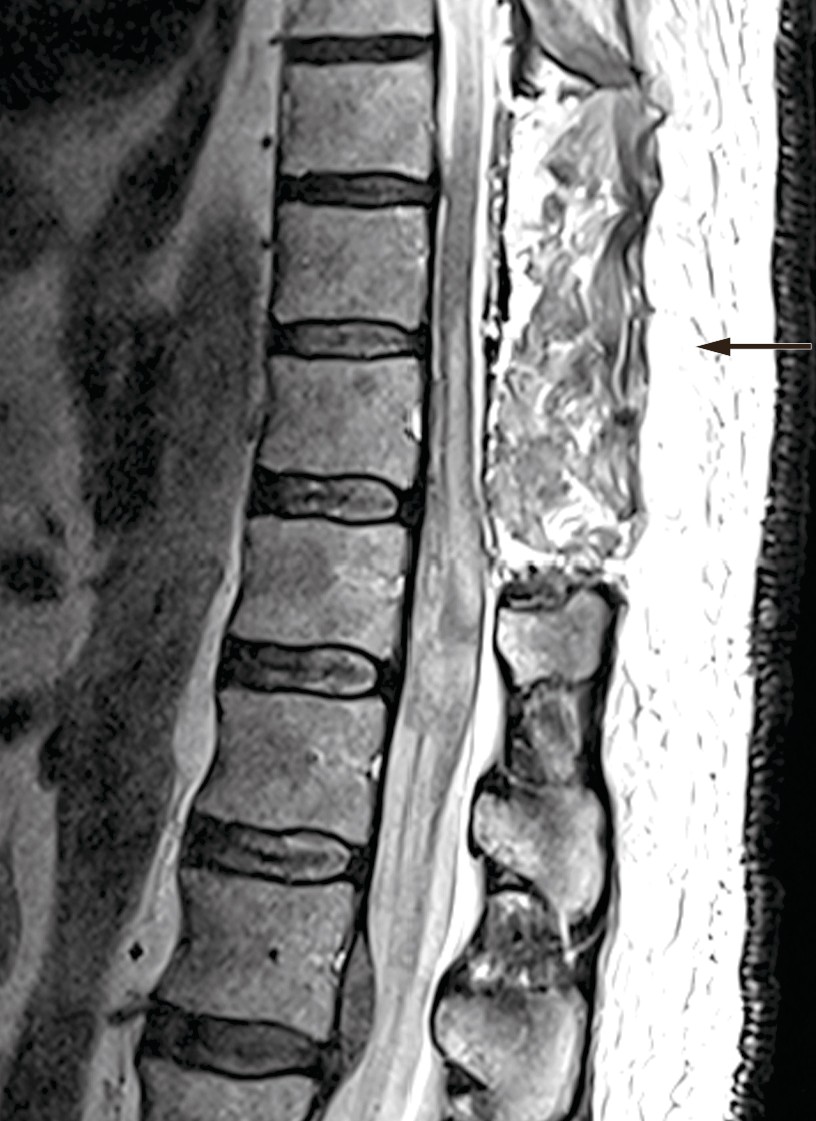
Figure 2 Postoperative MRI: Laminectomy performed at levels Th9–11. MRI shows reduction in volume of the subdural haematoma at the operated level, unchanged subdural haematoma at level Th12, and new intramedullary signal changes at levels Th10–L1.
A German meta-analysis on spinal cord haemorrhage showed a clear relationship between the time from symptom onset to surgical treatment and the reduction in neurological symptoms postoperatively. When surgery was performed within 12 hours, 66 % of patients achieved complete remission (5). Our patient underwent surgery 11 hours after symptom onset and was thus assumed to have a good prognosis.
The patient showed no improvement in neurological status postoperatively. Treatment with dalteparin (Fragmin, 5 000 IU subcutaneously × 1) was started on postoperative day one, and he received standard treatment in intensive care with mean arterial pressure (MAP) above 85 mm Hg the first week (7). MRI was repeated after ten days and showed a new epidural cerebrospinal fluid accumulation at level Th10–11 with compression of the spinal cord (Figure 3). MRI of the head was also performed on account of headache and nausea. This showed a small amount of intraventricular and subarachnoid blood, which was interpreted as having spread from the spinal cord haemorrhage.
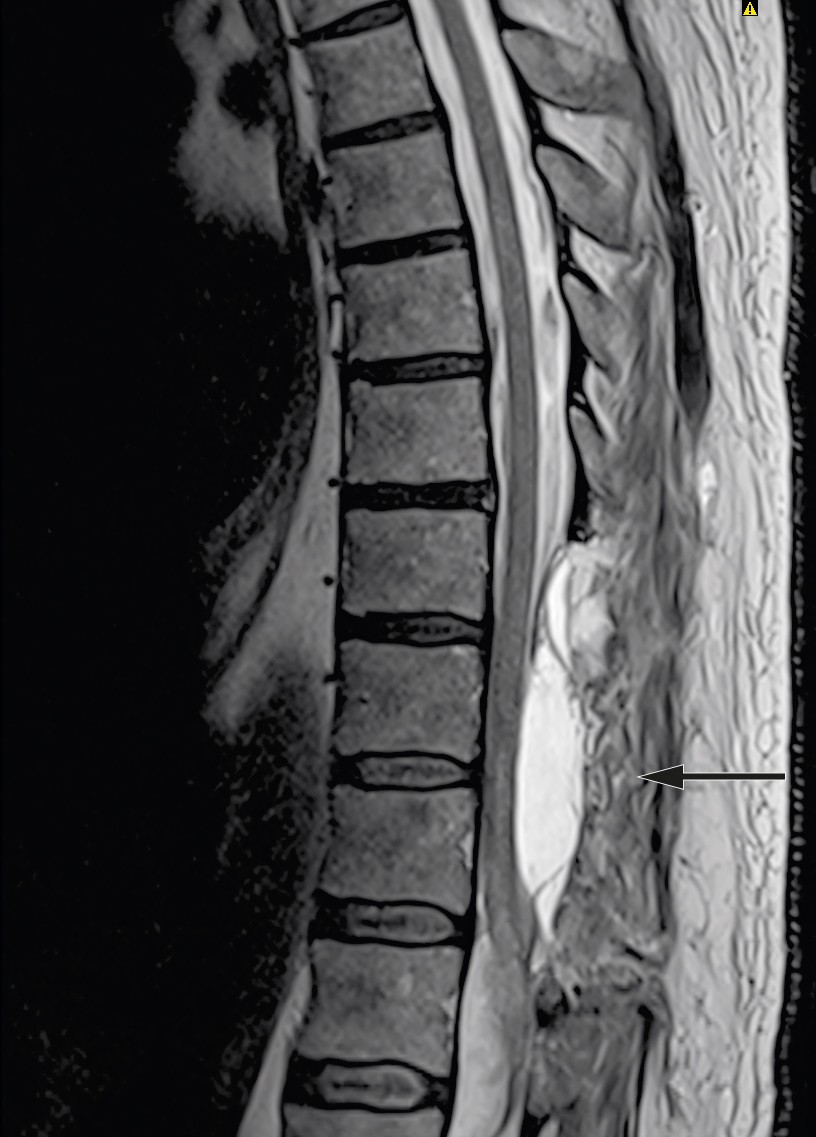
Figure 3 MRI ten days after surgery: New epidural accumulation of cerebrospinal fluid with mass effect.
Persistent neurological deficits with paralysis and loss of sensation, as well as loss of control over urination and defecation, indicated a need for specialised rehabilitation. Eighteen days after symptom onset, the patient was transferred to the spinal injuries unit. Dalteparin was then replaced with rivaroxaban (Xarelto, 20 mg × 1) owing to the patient’s history of pulmonary embolism and atrial fibrillation. Upon transfer, both legs were paralysed with reduced sensation from level Th11. His reflexes were extinguished, as expected in the initial phase of spinal shock. In spinal shock, flaccid muscle paralysis and hyporeflexia will be present for the first four to eight weeks after injury, despite damage to the central nervous system. Following resolution of the spinal shock, his reflexes returned and he developed spastic paresis. Motor function improved by 2–4 points in the right leg and 0–2 points in the left leg according to international classification criteria (8). Sensory function also gradually improved up to level Th12. The patient experienced neuropathic pain during his hospital stay, but did not need pain relief upon discharge. He had persistent neurogenic urinary bladder and bowel dysfunction and upon discharge he had to undergo self-catheterisation to empty his bladder and laxatives to achieve bowel movements at regular times. He required a manual wheelchair for all transfers, but was self-reliant with respect to daily chores. After just over three months of rehabilitation, he was discharged to specially adapted housing.
The patient was referred back to the university hospital when his clinical progress stagnated four months after the incident. MRI of the spine showed an increase in size of the cerebrospinal fluid loculus at Th7–11, in addition to the previously observed signal changes in the lumbar spinal cord and medullary cone. Despite additional surgery seven months after the incident, with laminectomy of Th12 and fenestration and emptying of an arachnoid cyst at Th11–12, the patient achieved no further clinical improvement. Treatment with rivaroxaban was reinstated postoperatively.
Five months after the incident, the patient undertook the first of five stays at a rehabilitation centre. He had sequelae after the spinal cord injury at level Th12, with reduced strength, mobility and function in both lower extremities, but particularly the left. The long-term goal of rehabilitation was to restore walking ability. The rehabilitation focused on improving his ability to stand via training in a standing frame, stimulating muscles in the lower extremities with TEMS (transcutaneous electrical muscle stimulation), and exercises to activate nerve and muscle function in the lower extremities. The training included cycling with motor assistance, as well as walking on a treadmill with bodyweight relief achieved by supporting the pelvis and both lower extremities. The patient has made progress in increasing his strength, especially on the right side, and has achieved a slight reduction in muscle tone. He now lives alone in his own apartment, uses a wheelchair, drives a car, performs exercises at home twice a week, and receives help from a sister with cleaning and shopping. He has normal bowel movements and performs self-catheterisation, but has pain and spasms in the legs that are treated with pregabalin (Lyrica), gabapentin (Neurontin) and baclofen (Baklofen).
Discussion
In common with the brain, the spinal cord can also be affected by infarction and haemorrhage, with haemorrhages accounting for approximately 15 % of such events (6). Spinal cord haemorrhage is rare and its incidence is unknown. We conducted a literature search in PubMed, but were unable to find the incidence of spinal cord haemorrhage, only that of spinal cord infarction, which is 3.1 per 100 000 person-years (9). In comparison, the Norwegian Spinal Cord Injury Registry recorded 125 spinal cord injuries in 2018, of which 48 % were non-traumatic injuries and 8.8 % had vascular aetiology (10).
As in the brain, spontaneous haemorrhages can also occur in the spinal cord. One third of these are idiopathic, but anticoagulation therapy, vascular malformations and spinal cord bleeding disorders are common causes. The association between spinal cord haemorrhage and anticoagulation therapy with warfarin or enoxaparin (Klexane) is recognised in the literature (3, 6). Arrhythmia and hypertension are predisposing factors; anticoagulation therapy on top of this increases the risk of spinal cord or epidural haemorrhage (5).
Our patient had several risk factors for spinal cord haemorrhage: anticoagulation therapy, paroxysmal arrhythmia and hypertension. Some countries include bleeding risk in the red flags for back pain, e.g. Canada, where coagulopathy is a red flag (11), but the Norwegian clinical guidelines for management of low back pain neither include nor mention anticoagulation therapy with respect to back pain (2). This may be because spinal cord haemorrhage is rare, and because the guidelines are aimed at multidisciplinary healthcare professionals: doctors, physiotherapists, chiropractors, osteopaths, naprapaths and nurses.
The guidelines for red flags in low back pain are consensus-based (1). Analysis shows that most red flags have low specificity and a low likelihood ratio for revealing serious pathology, and that it is the combination of several red flags that helps draw attention to possible underlying disease (12). As red flags are not research-based, their usefulness in screening is subject to debate; one American review claims that in back pain, red flags are probably more relevant for prognosis than for detecting serious underlying pathology (13). Screening with red flags can thus provide a false sense of security. The gold standard for detecting rare cases of serious pathology will probably always be a good medical history, targeted clinical examination, robust medical knowledge and knowledgeable follow-up.
Anticoagulation therapy increases the risk of bleeding, and the increasing use of direct-acting oral anticoagulants (DOACs) may thus pose a challenge for clinicians. As of yet, there have been only four case reports of spinal cord haemorrhage related to rivaroxaban, and only one related to dabigatran (Pradaxa). Spinal cord haemorrhage associated with the use of direct-acting oral anticoagulants appears to mainly involve the cervical and thoracic spine (80 %), but this remains uncertain owing to the small number of cases reported (14).
Our patient had classic symptoms of spinal cord haemorrhage. The pain was acute and was localised to the level of the bleeding in the spinal cord. In some cases, the pain is followed by pain-free intervals. Loss of strength and sensation then follows, depending on the degree of compression of the spinal cord. A meta-analysis showed that in addition to acute back pain, 30 % of patients had bladder dysfunction or complete paralysis of the bowel (5).
The decision on whether to operate is made by neurosurgeons, and depends on the aetiology and on the clinical status of the patient. Bleeding may be epidural, subdural, subarachnoid or intramedullary, and determining the exact location of the bleeding on MRI can be difficult. Patients with epidural or subdural haemorrhage and neurological deficits should be strongly considered for emergency surgery (15, 16). When spinal vascular lesions are the source of the bleeding, interventional radiology may be considered in addition to surgery. In the absence of vascular lesions, the standard surgical strategy is decompression and gentle evacuation of the haematoma. Regardless of aetiology, the prognosis is uncertain if the patient has severe postoperative neurological deficits.
Intraspinal haemorrhage with neurological deficits and the need for rehabilitation is considered a form of non-traumatic spinal cord injury. In Norway, these patients will therefore fall within the target group for the country’s spinal cord injury units, which are located at St. Olavs Hospital (Trondheim), Haukeland University Hospital (Bergen), Oslo University Hospital and Sunnaas Rehabilitation Hospital (Oslo area). Relatively little is known about the course of intraspinal haemorrhage and the effects of rehabilitation, but a few case studies and literature reviews have been published (17, 18). The course in our patient is consistent with a number of published case studies, in which patients with intraspinal haemorrhage without complete loss of motor and sensory function experienced a partial recovery of neurological function, but often had some degree of sequelae. Following specialised rehabilitation, many patients will benefit from further follow-up at rehabilitation centres and by the primary healthcare service.
This case report illustrates that spinal cord haemorrhage should be considered in patients with a history of coagulopathy or undergoing treatment with anticoagulants, who develop acute neck or back pain. It is important to strive for rapid admission to the neurosurgical department because the duration of symptoms correlates with prognosis after surgery. Although anticoagulation therapy and coagulopathies increase the risk of spinal cord haemorrhage, these factors are not included among the red flags in the Norwegian clinical guidelines for management of low back pain, which may lead to spinal cord haemorrhage being overlooked as a cause of back pain. We believe anticoagulation therapy should be included among the red flags in the aforementioned guidelines. This could be done either by introducing ‘Coagulopathy and use of anticoagulants’ as a separate point, or by adding the information to an existing point: “Trauma, tumour, use of anticoagulants, steroids or immunosuppressants, substance abuse’.
