A woman in her forties who was hospitalised after a three-week history of respiratory tract infection, developed severe acute respiratory distress syndrome. Nasopharyngeal swabs taken on days 1 and 3 after admission were negative for the SARS-CoV-2 virus, while bronchoalveolar lavage tested positive. We assume this is because the patient had stopped viral shedding in the upper respiratory tract because of the long time between symptom onset and testing.
An active woman in her forties was hospitalised with a three-week history of illness with influenza-like symptoms and fever. The patient had a diagnosis of type II diabetes mellitus which was diet-regulated, and hypothyroidism. Twenty years earlier, the patient had been diagnosed with moderate obstructive ventilatory impairment with FEV1 (forced expiratory volume after one second) of about 65 % of the expected level. This was unchanged at her last spirometry test ten years ago. The patient had been a daily smoker for 25 years and had stopped smoking seven years prior to her hospitalisation. She was receiving treatment for multiple sclerosis with the potentially immune-suppressing drug rituximab, with infusion every six months. The previous infusion had been administered four months prior to her hospitalisation.
The patient had been self-isolating at home since the onset of symptoms because of the COVID-19 epidemic. The last 2–3 days before hospitalisation her general condition and intake of solids and fluids had been reduced. There was no report of nausea, vomiting, pain or a change in sense of taste. On admission the patient was lethargic and confused. In the ambulance her blood pressure while supine was measured by her GP as 117/76 mm Hg and her temperature as 39.2 °C (assumed tympanic temperature). On admission her respiratory rate was about 30 per minute and oxygen saturation with 5 l oxygen given by mask about 90 %. Her heart rate was about 100 per minute, and non-invasive blood pressure was measured as 101/74 mm Hg in slight Trendelenburg position and with ongoing fluid therapy. Her tympanic temperature was 37.7 °C. The chest x-ray showed extensive patchy opacities bilaterally which, coupled with the clinical picture, led to a diagnosis of acute respiratory distress syndrome (ARDS). Despite the patient’s confused state, no head CT or lumbar puncture was performed, as the symptoms were considered secondary to severe pneumonia. There were no other neurological symptoms. We assume that uncertainty at the time as to whether suspected SARS-CoV-2 infection should be isolated with droplet or airborne precautions, may have influenced the decision not to perform a CT scan.
Laboratory analyses from admission tests are shown in Table 1. The patient had metabolic alkalosis, which may have been due to respiratory failure over a period of time, with renal compensation. The patient appeared hypovolaemic, and compensatory hyperaldosteronism may also have contributed to alkalosis and hypokalaemia. Off-label antiviral therapy was administered in the form of lopinavir/ritonavir and hydroxychloroquine. Because of the patient’s critical condition, treatment with cefotaxime and ciprofloxacin was initiated against possible secondary bacterial pneumonia, after specimens had been secured.
Table 1
Results of blood tests on admission to hospital. Outliers are in bold print
|
Analysis
|
Admission date
|
Reference range (adults)
|
|
Haemoglobin (g/100 ml)
|
13.7
|
12.0–14.7
|
|
Leukocytes (· 109/l)
|
4.5
|
3.9–9.5
|
|
Differential count, automated (· 109/l)
|
|
|
|
|
Neutrophil granulocytes
|
3.6
|
1.5–5.7
|
|
|
Lymphocytes
|
0.7
|
1.3–3.4
|
|
|
Monocytes
|
0.13
|
0.31–0.92
|
|
|
Eosinophil granulocytes
|
0.00
|
0.00–0.40
|
|
|
Basophil granulocytes
|
0.01
|
0.00–0.10
|
|
Platelets (· 109/l)
|
300
|
145–390
|
|
Erythrocytes (· 1012/l)
|
4.9
|
3.9–5.1
|
|
MCV (fl)
|
85
|
84–97
|
|
APTT (sec .)
|
40
|
30–42
|
|
CRP (mg/l)
|
123
|
0–4
|
|
PT-INR
|
1.3
|
0.8–1.2
|
|
Fibrinogen (g/l)
|
6.1
|
2.0–4.0
|
|
D-dimer (mg/l)
|
2.9
|
0.0–0.4
|
|
Albumin (g/l)
|
29
|
36–45
|
|
Lactate dehydrogenase (U/l)
|
820
|
105–205
|
|
Creatinine (µmol/l)
|
58
|
45–90
|
|
Estimated GFR (ml/min)
|
106
|
|
|
Bilirubin total (µmol/l)
|
17
|
5–25
|
|
Alkaline phosphatase (U/l)
|
50
|
35–105
|
|
Amylase (U/l)
|
25
|
25–120
|
|
AST (U/l)
|
35
|
15–35
|
|
ALT (U/l)
|
15
|
10–45
|
|
GT (U/l)
|
25
|
10–75
|
|
Troponin T (ng/l)
|
< 5
|
0–14
|
|
Potassium (mmol/l)
|
3.3
|
3.5–5.1
|
|
Sodium (mmol/l)
|
137
|
137–145
|
|
Chloride (mmol/l)
|
96
|
95–105
|
|
Ionised calcium, (mmol/l)
|
0.82
|
1.14–1.28
|
|
Arterial blood gas
|
|
|
|
|
pH
|
7.51
|
7.35–7.45
|
|
|
Actual bicarbonate (mmol/l)
|
42.1
|
22.0–26.0
|
|
|
pO2 (kPa)
|
7.4
|
11.0–14.0
|
|
|
pCO2 (kPa)
|
7.3
|
4.5–6.0
|
|
|
Lactate (mmol/l)
|
1.9
|
0.5–1.6
|
|
|
Base excess (mmol/l)
|
19
|
-3–3
|
|
|
Glucose (mmol/l)
|
22.4
|
4.0–6.0
|
The patient was isolated with droplet precautions in the Intensive Care Unit because of suspected COVID-19 infection, and a nasopharyngeal swab was taken for a polymerase chain reaction (PCR) assay for SARS-CoV-2. Given the existing clinical picture, the patient would normally have been treated with non-invasive ventilation (NIV): a mask that fits tightly over the nose and mouth is placed on the patient, and the respiration is assisted by a ventilator. This treatment was not given, as it was assumed that the spread of aerosols in the room could increase the risk of infection for the staff. After 18 hours the patient’s condition had deteriorated, with a respiration rate of about 40 per minute and peripheral oxygen saturation of 90 % with 10 l oxygen by non-rebreather mask. With the staff dressed with airborne precautions and using FFP3 half-masks fitted, with filters to provide the highest level of protection against particles, the patient was intubated without complications. Because of her persistent high oxygen requirement after intubation ((pO2(a)/FiO2-ratio < 10) the patient was placed in the prone position. The first three days after intubation the patient was given an infusion of muscle relaxant (cisatracurium) to improve patient-ventilator interaction and at the same time limit the risk of infection. All connections between the endotracheal tube and ventilator were thoroughly taped to avoid the spread of aerosols in the air if disconnection should take place by accident (Fig. 1).
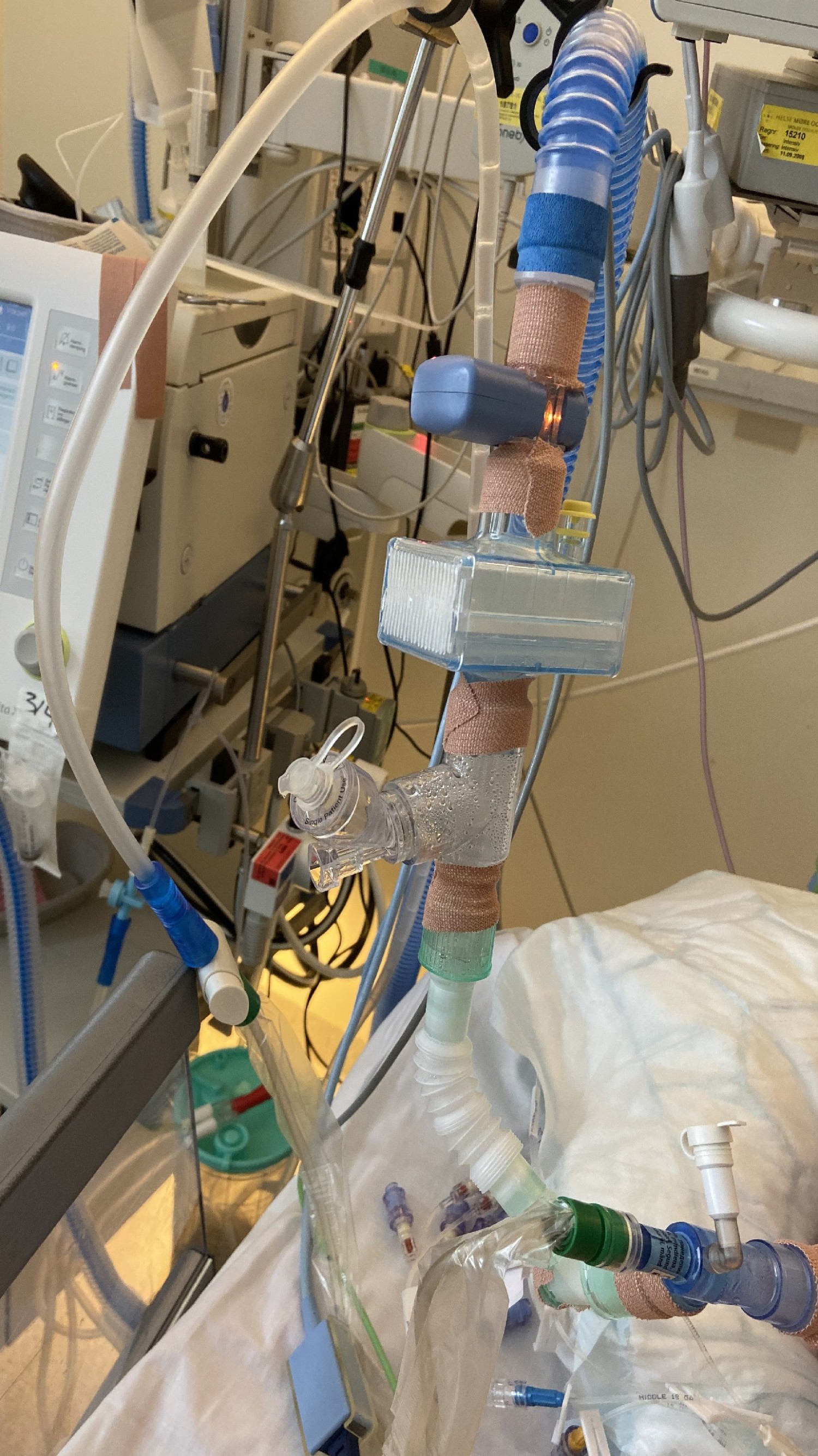
Figure 1 Taping of ventilator connections to prevent the spread of aerosols in the room in the event of accidental disconnection
The first nasopharyngeal swab was negative for SARS-CoV-2, but because of the strong clinical suspicion of coronavirus pneumonia, bronchoalveolar lavage (BAL) was performed. This was virus-positive in a PCR assay for the betacoronavirus E-gene. Exponential amplification started after 16 cycles (Ct value), which equates to a very high viral load. A new nasopharyngeal swab was taken about 40 hours after admission was also negative. The patient was ventilated according to the guidelines for treatment of acute respiratory distress syndrome, and she needed to be in a prone position about 16 hours a day for seven of the first eight days of her intensive care treatment (1). She was given 45–55 % oxygen in inspiration air during this period, with oxygen saturation of around 90 % and a positive end-expiratory pressure (PEEP) of 12–14 cm H2O. During the disease course she had normal renal function, but temporary liver failure and high serum ferritin with a maximum level of 1 189 µg/l (reference 23–431 µg/l) on day 10. The patient was extubated on day 14, but because of laryngeal oedema and stridor she was re-intubated, and surgical tracheostomy was performed the same day. On day 18 she was decannulated. At the time of publication, the patient has been transferred to the pandemic ward and her condition is improving.
Discussion
The fact that the patient tested negative for SARS-CoV-2 in two nasopharyngeal swabs, but had very high viral loads in bronchoalveolar lavage fluid, may seem surprising. However, it has been shown that the viral load in the upper respiratory tract is high in the first few days after the onset of the disease, and then falls rapidly (2, 3). When a patient contracts viral pneumonia, the levels may be low and undetectable. It is important that health personnel and laboratories are aware of this, because in light of the negative nasopharyngeal tests, a reduction of isolation measures was considered. This would have resulted in a significantly higher risk of spread of infection among the personnel involved in the treatment, and several of them would have been quarantined. It is also uncertain to what degree the patient was immunosuppressed due to treatment with rituximab, and whether this, coupled with the underlying pulmonary disease, caused critical illness.
A number of therapeutic considerations had to be balanced against the risk of infection to the personnel. The patient did not receive non-invasive ventilation prior to intubation, despite clinical indication. Weaning from the ventilator was complicated by the fact that non-invasive ventilation was not considered an option after extubation, which lowered the threshold for tracheostomy. In order to limit the risk of accidental extubation or disconnection from the ventilator, the patient received deeper sedation than would have been given under normal conditions. Accidental disconnection of the ventilator when the personnel were not sufficiently protected would have implied a risk of infection and quarantine, with consequences for the staffing. Changing between prone and supine position twice a day increased the probability for such events. At the start of the treatment, there were not sufficient FFP2 and FFP3 masks available for everyone to wear during these types of procedures, which gave rise to several discussions between the healthcare personnel. The patient was also given an infusion with a muscle relaxant for one day more than recommended, in order to improve ventilator cooperation and to reduce the risk of contamination. However, we do not believe this to have had any long-term consequences for the patient.
We started the process of publishing this case report with the consent of the patients´ next of kin, before the patient was competent to give informed consent. The ethical grounds for doing so were infection control considerations, as false negative nasopharyngeal tests may affect diagnostic considerations if the clinical evidence otherwise points to viral pneumonia due to SARS-CoV-2.
This was the first ventilator patient with COVID-19 in our hospital. As a result, infection control considerations had to be balanced against treatment quality. We hope our case report may be useful for the readers of the Journal of the Norwegian Medical Association, not least the fact that negative nasopharyngeal tests after a prolonged history of illness do not necessarily exclude SARS-CoV-2 viral pneumonia.