Odontogenic infections are infections that originate in the teeth and/or their supporting tissues. Such infections are common, and a large proportion of infections of the head and neck region are of odontogenic origin. Most odontogenic infections cause mild signs and symptoms, but they can also develop into serious conditions.
This article provides an overview of the most common pathogenic microbes in the oral cavity, the most frequently occurring odontogenic infections, and the treatment and potential complications of the latter. The article is based on a non-systematic search in PubMed, plus the authors’ own clinical experience and literature archives.
Causative microbes
The oral cavity contains more than 700 naturally occurring species of bacteria. One of the most widespread bacterial genera in the oral cavity is streptococcus. Common species include S. mitis, S. sanguinis, S. salivarius and S. anginosus, and these may contribute to the development of caries, marginal periodontitis and endocarditis (1).
The bacteria in the oral cavity are opportunists that can cause infection if the interaction between host and microbe changes. In the early phase of an infection, the causative microbes will reflect the normal flora in the oral cavity, but as the infection progresses, anaerobic species will usually predominate.
In addition to causing local infections in bone and soft tissues, oral bacteria can contribute to the development of Alzheimer’s disease, endocarditis, atherosclerosis, osteomyelitis of non-craniofacial bones, rheumatoid arthritis and diabetes mellitus. Studies have also shown that maternal marginal and apical periodontitis may be associated with low birth weight of newborns (2–5).
Typical bacterial odontogenic infections
Marginal periodontitis
The gum disease marginal periodontitis is an inflammatory condition that affects the periodontium, the supportive tissues of the teeth. Studies have shown that approximately 50 % of adults in the USA have marginal periodontitis, and that 8 % of 35-year-olds in Oslo have advanced periodontal destruction (6, 7).
The association between marginal periodontitis and diabetes is well documented, with patients with either condition at increased risk of developing the other (8). Studies have shown that there is also an association between marginal periodontitis and cardiovascular disease, but there is currently insufficient evidence to indicate a causal relationship (8).
Marginal periodontitis is caused by the accumulation of a biofilm (plaque) along the gum line. This gives rise to a superficial infection of the gingiva (gingivitis) which can lead to a deeper infection along the root of the tooth (marginal periodontitis). The condition is progressive and may eventually cause tooth loss. Smoking, diabetes and stress are known risk factors (8).
Treatment of marginal periodontitis is performed by dentists or dental hygienists and consists of mechanical removal of plaque and tartar. Antibiotics are indicated in some cases of aggressive periodontitis.
Apical periodontitis
The root canal is essentially a sterile, closed system. However, bacteria can enter the canal via caries, fractures, cracks between a filling and a tooth, and periodontal pockets. The ensuing immune response with recruitment and activation of leukocytes leads to inflammation of the dental pulp (pulpitis), which can be very painful. If left untreated, bacteria can invade the dental pulp and cause necrosis. Infection of the dental pulp will in turn lead to inflammation of the periodontal ligament, and bacterial products may exit the root canal through the root apex. The subsequent immune response will lead to inflammation and destruction of periapical tissue, so-called apical periodontitis (9).
The condition is usually asymptomatic. However, the production of pus can result in the formation of a periapical abscess (Figure 1). This usually causes pain, swelling and occasionally fever. Periapical infections can also spread to surrounding structures (Figure 2). Draining of pus via a fistula (extra- or intraoral) will reduce symptoms. Acute periapical abscesses must be rapidly drained of pus, either via the root canal or via an incision. This procedure is primarily performed by dentists.
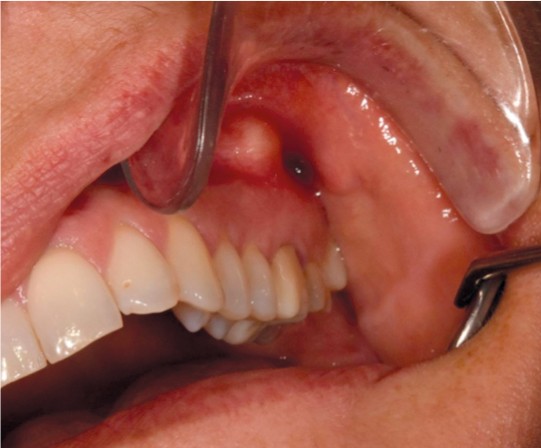
Figure 1 Periapical abscess from upper left molar.
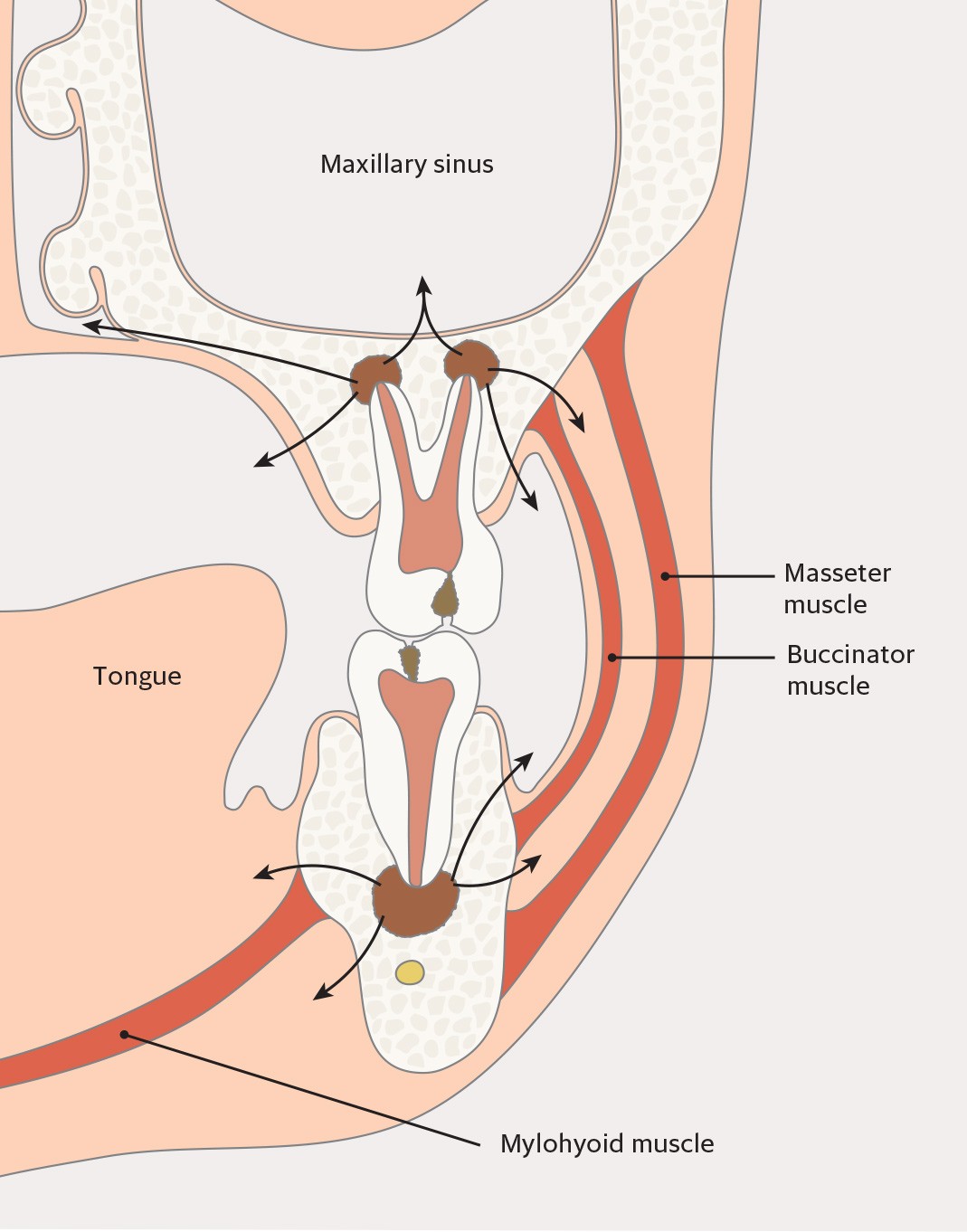
Figure 2 Possible routes of spread of periapical infections from upper and lower molars.
If the patient shows signs of systemic effects with reduced general condition, he or she should also be treated with antibiotics. In addition, the focus of infection must be removed, preferably within a few days, by cleaning the root canal via endodontic therapy (i.e. root canal treatment) or by extracting the tooth. The same treatment principles apply for abscesses with an established fistula, where treating the focus of infection will lead to regression of the fistula.
Pericoronitis
Pericoronitis is an infection that originates in the soft tissue around partially retained teeth (teeth that remain in the jaw). The condition occurs mainly in early adulthood, and usually affects wisdom teeth in the lower jaw when food debris and other foreign matter accumulates between the gums and the partially erupted crown of the tooth. This typically leads to a low-grade, recurring infection with pain, swelling and redness in the surrounding gingiva. In some cases, patients may also have trismus (lockjaw), fever and reduced general condition.
Predisposing factors are stress, menstruation and recent or current illness. Pericoronitis is usually self-limiting, but recurrence is common until the tooth erupts or is extracted. An abscess may also form in some cases, necessitating incision and drainage. Teeth with recurrent pericoronitis should usually be extracted during an inactive phase of the infection.
Other bacterial infections with possible odontogenic aetiology
Odontogenic sinusitis
Up to 40 % of all maxillary sinusitis is of odontogenic origin (10). The most frequent causes of odontogenic maxillary sinusitis are apical periodontitis, marginal periodontitis and oroantral fistulas following extraction of upper molars (10). The condition typically results in pain, mucosal oedema and nasal secretion. In contrast to rhinogenic sinusitis, maxillary sinusitis is usually unilateral. Treatment consists of removing the focus of the dental infection, with elective functional endoscopic sinus surgery (FESS) if indicated. If the sinusitis is caused by a persistent oroantral fistula following tooth extraction, the fistula may be surgically closed by a specialist in maxillofacial surgery (medical specialty) or oral surgery (dental specialty).
Osteomyelitis
Acute purulent bacterial osteomyelitis can occur secondary to infections of the teeth and the periodontium, if the infection has spread to cortical and cancellous bone. The lower jaw is most frequently affected and patients often have severe pain, fever, discharge of pus from the area, trismus and halitosis owing to the presence of anaerobic bacteria (11). Paraesthesia or hypoaesthesia of the lower lip and chin may also occur if the inferior alveolar nerve is affected. If treatment is delayed, acute osteomyelitis may turn into chronic disease. Chronic osteomyelitis usually has milder symptoms than the acute form and is characterised by sequestration and possibly fistulation (11).
Computed tomography (CT), panoramic dental x-ray (orthopantomogram, OPG) and magnetic resonance imaging (MRI) can reveal varying degrees of osteolysis, osteosclerosis and sequestration. Treatment consists of antibiotics, drainage of any abscess, and possibly surgical revision by a maxillofacial or oral surgeon with removal of infected, necrotic tissue. The source of the infection must also be managed, for example through endodontic therapy or the extraction of one or more teeth.
Necrotising fasciitis
Necrotising fasciitis is a serious, destructive and progressive soft tissue infection that can quickly become life-threatening. The disease is divided into two subtypes based on the causative bacteria. Type 1 is caused by streptococci (not group A), enterobacteria or obligate anaerobes, and type 2 is caused by group A streptococci (12).
The condition begins with thrombosis in small blood vessels in the periphery of the focus of infection, leading to acute inflammation and to oedema in subcutaneous tissues and possibly the skin (Figure 3). Necrosis then occurs in the infected tissue. This spreads rapidly along the superficial fascia, nerves, arteries and veins (12). Left untreated, the majority of patients will develop sepsis within 48 hours (12). Owing to its rich vascular supply, necrotising fasciitis rarely occurs in the head and neck area, with a reported annual incidence of only 2 cases per 1 000 000 population (12). The most common cause of necrotising fasciitis in the head and neck area is odontogenic infection, but the condition can also occur secondary to pharyngitis, tonsillitis, acute otitis media and dermatological infections (12). If an odontogenic origin of necrotising fasciitis is suspected, the patient should be referred immediately to a maxillofacial surgery department or alternatively to the nearest department of otorhinolaryngology or plastic surgery.
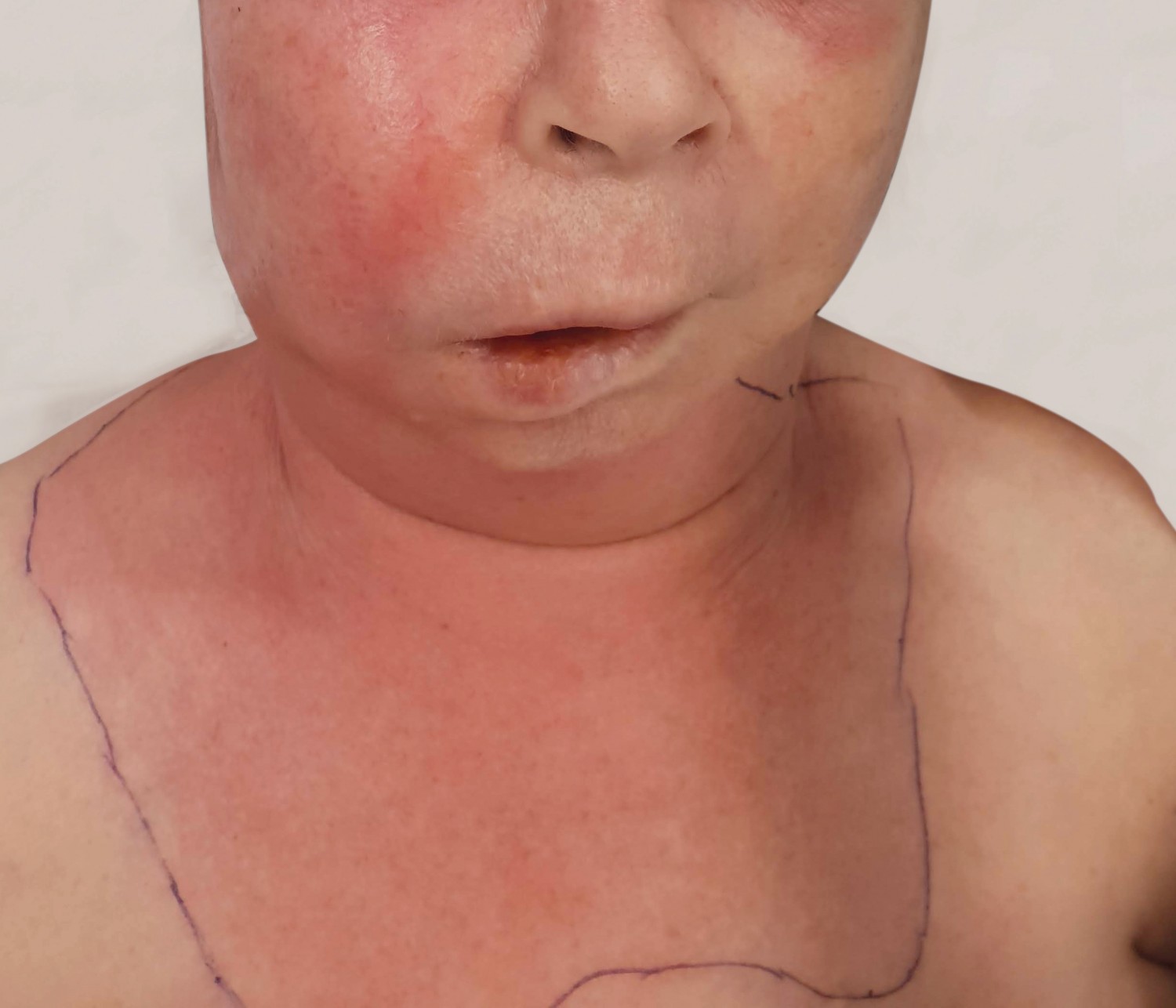
Figure 3 Necrotising soft tissue infection originating from a periapical abscess on an upper right molar.
Contrast CT is necessary to determine the location and extent of the infection, as well as to reveal any primary focus, for example periapical lucencies on tooth roots. Contrast CT can furthermore show asymmetric fascial thickening, gas bubbles along the fascial planes, oedema of muscle and soft tissues, and possibly fluid accumulation in fascial spaces indicating an abscess (13). Prompt treatment with radical surgical debridement of necrotic tissue and antibiotics is important. Norwegian national guidelines recommend combination therapy with cefotaxime and metronidazole with the addition of clindamycin if indicated (14).
Ludwig’s angina
Ludwig’s angina was first described by the German physician Friedrich von Ludwig in 1836, and is a rapidly progressive infection of the submandibular and sublingual spaces. The infection does not usually result in abscess formation, but is phlegmonous and spreads diffusely over both sides of the floor of the mouth. Patients usually have reduced general condition, fever and pronounced submandibular swelling. The tongue may become swollen and can be pushed out of the mouth, into the retropharyngeal space or up against the palate, leading to upper airway obstruction. The infection may also cause airway obstruction by spreading to the lateral neck compartment or retropharyngeal space.
Left untreated, the condition has a mortality rate of around 50 %, and vigilance is necessary with respect to protecting the airway (15). Patients must therefore be referred urgently to a hospital with a maxillofacial surgeon or otorhinolaryngologist. Odontogenic infections of the lower second and third molars are the most common cause of Ludwig’s angina, because their roots are caudal to the mylohyoid muscle (15). Other precipitating factors include peritonsillar or parapharyngeal abscesses, infection secondary to a mandibular fracture, or submandibular sialadenitis (15). CT with intravenous contrast is recommended to determine the extent of the infection and whether an abscess is present. Rapid diagnosis is important to enable prompt initiation of antibiotic therapy along with incision and drainage in cases of purulent infection. Some also recommend the use of systemic steroids (16).
Lemierre’s syndrome
If an odontogenic infection spreads dorsolaterally to the lateral neck compartment, the result can be septic thrombophlebitis of the internal jugular vein. The condition is rare, with annual incidence reported to be 3.6 cases per 1 000 000 population (17). However, the number of published case reports is increasing, probably due to greater awareness of the condition as well as improved diagnostic imaging (17). The syndrome most often affects previously healthy children and young adults.
The most common aetiology is a recent episode of viral pharyngitis. This probably compromises the mucosal barrier, leading to the migration of bacteria into deeper tissues. Opportunistic gram-negative rods in the form of fusobacteria have been described as the most common causative agents, but up to one-third of cases are polymicrobial (17). Septic embolisation occurs in the majority of cases, most commonly in the lungs and large joints, but septic emboli in the brain, skin, liver and pericardium have also been reported (17). In addition to signs and symptoms resulting from any emboli, patients often have unilateral neck pain, fever, trismus, a palpable mass corresponding to the internal jugular vein, and positive blood cultures.
Treatment consists of long-term antibiotic therapy, which has improved the prognosis from a mortality rate of 90 % in the pre-antibiotic era to less than 18 % in modern times (17, 18). The use of anticoagulation is controversial. Depending on the aetiology, cases of Lemierre’s syndrome should be discussed with a department of maxillofacial surgery or otorhinolaryngology.
Spread of infection to deep cervical fascial compartments
The spread of infection to the deep neck spaces can have serious consequences. There are at least 11 deep neck spaces defined by the various fasciae of the face and neck. The majority of deep neck infections result from the spread of primary odontogenic infections (19). Spreading follows the path of least resistance along the fascia, and can lead to cavernous sinus thrombosis, cerebral abscess, meningitis, mediastinitis or pericarditis (19).
Signs and symptoms depend on the location of the infection. They may include fever, reduced general condition, swelling, pain upon swallowing, dysphagia, hoarseness, stridor and trismus. Swelling can lead to compression of the trachea, which can threaten airway patency. Contrast CT can reveal both the location of the infection and whether it is phlegmonous or abscessing. Prompt treatment with antibiotics is important, along with surgical drainage of pus if indicated. In common with Lemierre’s syndrome, the aetiology should determine where treatment takes place.
Conclusion
Uncomplicated local odontogenic infections are common and can usually be managed by a dentist. In some cases, odontogenic infections can develop into serious conditions with significant morbidity. It is therefore important to be aware of these conditions and to refer any suspected cases to hospitals with expertise in maxillofacial or otorhinolaryngological surgery.
