Colorectal cancer is the most prevalent form of cancer among Norwegian men and woman, with 4 428 new cases in 2018 (1). Since the 1940s there has been a doubling of incidence, but also of five-year relative survival, which is now at about 70 % (1). Higher survival is attributed partly to new treatment methods and enhanced surgical techniques, particularly for rectal cancer, and to adjuvant therapy (2).
Adjuvant chemotherapy is administered after surgery to prevent metastases and local recurrence. The Norwegian guidelines recommend three to six months of treatment with different combinations of 5-fluorouracil (5-FU), folinate, capecitabine and oxaliplatin for patients with stage III and high-risk stage II colon cancer. Studies show that the combination of 5-fluorouracil, folinate and oxaliplatin (so-called FOLFOX course) or capecitabine and oxaliplatin (CAPOX course) started within four to six weeks of surgery increase both five-year freedom from disease and overall survival by 2–4 % and 10–15 % for cancer in stages II and III (3). With rectal cancer, individualised chemotherapy is used, based on the patient’s risk factors (3).
Cytostatics have a low therapeutic index, and some patients with colorectal cancer experience adverse effects from adjuvant chemotherapy which lead to dose reduction, delayed treatment or discontinued treatment. The different drugs have different adverse effect profiles, which in turn depend on the dose and administration regimen and on individual differences in tolerance (4). Common terminology criteria for adverse events (CTCAE criteria) classify the various adverse events according to severity, from 1 to 5: mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening (grade 4) and death (grade 5). Grades 3 and 4 generally lead to a dose reduction or to postponement or discontinuation of the course of treatment (5).
The most common dose-limiting adverse effects of 5-fluorouracil are gastrointestinal side effects and bone-marrow depression (4). 5-fluorouracil and capecitabine also increase the risk of hand-foot syndrome, a condition involving pain and skin changes in the palms of the hands and soles of the feet. Combination with oxaliplatin often causes more pronounced bone marrow depression, in addition to dose-limiting neurotoxicity (4).
At present dosage of adjuvant chemotherapy is based on the patient’s surface area (see Table 1) (6). The dosage method is based on a formula derived by Du Bois and Du Bois on the basis of a study from 1916 with nine included patients (7). Several new formulae were derived from this, and the most widely used today is Mosteller’s formula of 1987 (8). Although the dosage method has been in use for many years, it has limitations, as it does not take into account either individual difference in body compositions or pharmacokinetics (9). It has been found that patients with the same surface area, body weight and body mass index (BMI) may have large differences in body composition (see Table 2 for explanation of terms) (9). The drugs in the chemotherapy regimen are largely water-soluble and distributed and metabolised first and foremost in fat-free tissues (10). An individual’s fat-free mass may therefore have a bearing on treatment efficacy and chemotherapy toxicity.
Table 1
Formulae for estimating body surface area for cytostatic dosage. BSA = body surface area, i.e. the estimated surface area of the body.
|
Authors
|
Formula
|
|
Du Bois and Du Bois (7)
|
BSA (m2) = weight (kg)0.425 ∙ height (cm)0.725 ∙ 0.007184
|
|
Mosteller (8)
|
BSA (m2) = weight (kg)0.5 ∙ height 0.5 ∙ 0.016667
|
Table 2
Explanation of terms.
|
Term
|
Explanation
|
|
Body mass index (BMI)
|
Weight (kg) / height (m)2
|
|
Fat mass
|
The body’s total adipose tissue
|
|
Fat-free mass
|
Consists of the body’s skeletal musculature and metabolic tissues such as liver, kidneys, intra-and extracellular fluid and bone tissue
|
|
Skeletal muscle mass index
|
Skeletal muscle mass area (cm2) / height (m)2
|
|
Psoas index
|
Psoas muscle area (mm2) / height (m)2
|
Several studies have identified a relationship between body composition and chemotherapy toxicity among patients with metastatic colorectal cancer (9). However, the results of these studies cannot necessarily be transferred to patients with localised disease. In this article we aim to summarise studies that have investigated the relationship between disease-related malnutrition and tolerance of adjuvant chemotherapy in patients with stages II–III colon cancer. We have also included studies that analyse colorectal cancer collectively, as the findings will largely be applicable to a pure colon cancer population.
Method
We searched in PubMed using MeSH terms that covered the Norwegian designations tykk- og endetarmskreft (colorectal cancer), adjuvant kjemoterapi (adjuvant chemotherapy), ernæringsstatus (nutritional status) and toksisitet (toxicity). Synonyms and supplementary search terms were added for each of these (Appendix 1). The search was concluded on 28.5.2019 and conducted without restrictions. From a total of 553 hits, 514 articles were excluded on the basis of title and abstract. The majority of them did not cover relevant patient populations or used non-relevant outcome variables. Thirty-nine articles were read in full text, and ten of these were included in the review (Figure 1). Literature search and selection of research articles were conducted by first authors Kværner and Harnæs in consultation with last author Blomhoff. The inclusion criteria were set up according to the PI/ECOTSS framework for framing research questions (Table 3). Studies that had investigated at least one symptom of disease-related malnutrition were included.
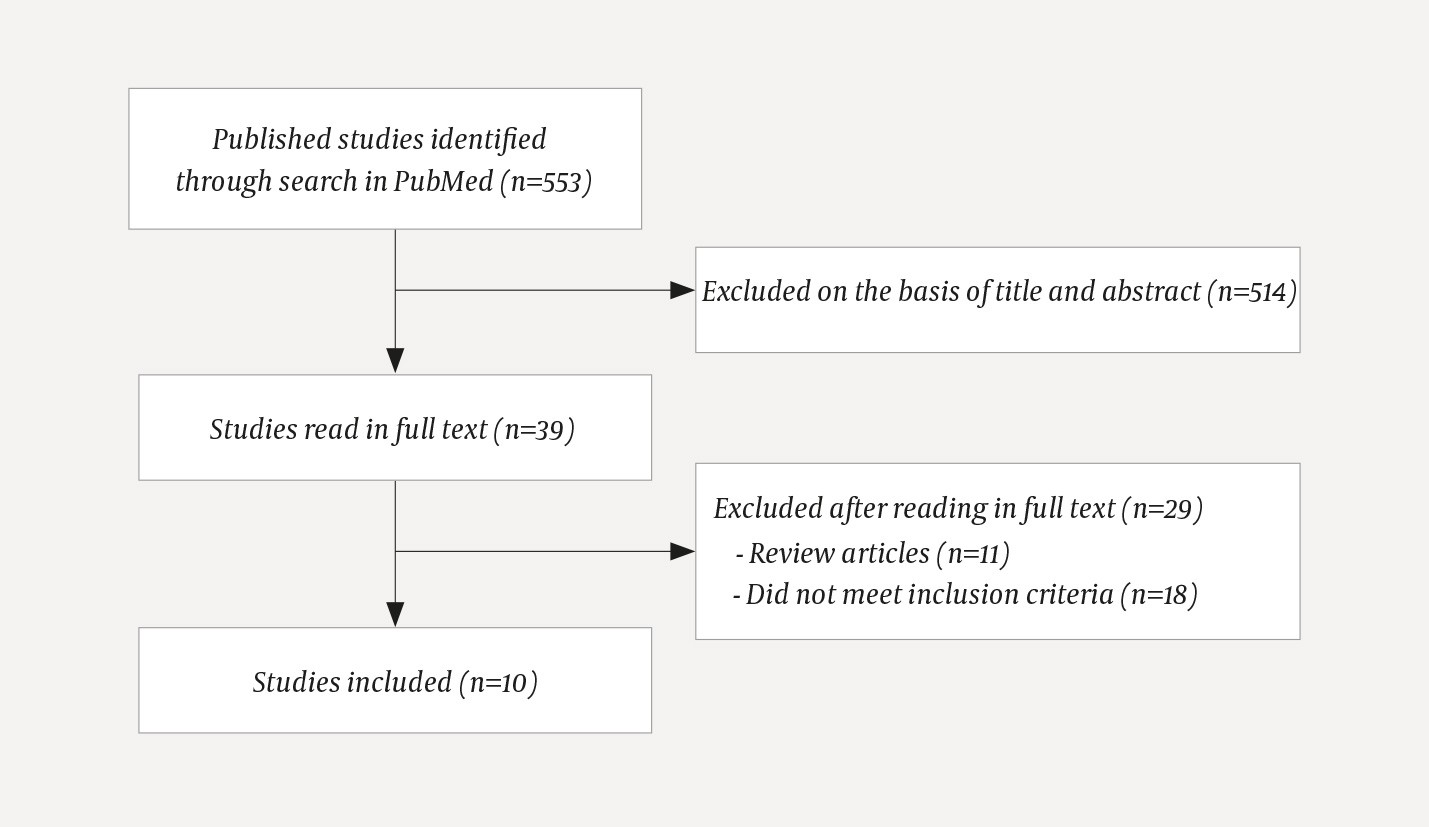
Figure 1 Flow chart for literature search and basis for selecting relevant studies
Table 3
Inclusion criteria according to the PI/ECOTSS framework.
|
Variable
|
Criterion
|
|
Population
|
Patients with stages II–III colorectal cancer who received adjuvant chemotherapy
|
|
Intervention/Exposure
|
At least one symptom of disease-related malnutrition according to the Global Leadership Initiative on Malnutrition (GLIM) criteria1
|
|
Comparator
|
No clinical signs of disease-related malnutrition
|
|
Outcome
|
Experience of dose-limiting toxicity, dose reduction, postponed dose or discontinued course
|
|
Timing
|
No restrictions
|
|
Setting
|
Relevant adjuvant chemotherapy (5-fluorouracil, folinate, oxaliplatin, capecitabine)
|
|
Study design
|
Intervention studies and observational human studies written in English or a Scandinavian language
|
Studies where parts of the patient population met all inclusion criteria were included. The results of these studies were restricted to patients who met the inclusion criteria, as far as possible. Review articles were not included, but their reference lists were checked without further findings.
Results
The results of the literature review are presented by symptom of disease-related malnutrition. For a complete overview and description of the included studies, including patient population, chemotherapy regimens, measurement methods, exposure variables and outcomes, see Appendix 2.
Relationship between low muscle mass and toxicity
Six studies found a direct or indirect association between low skeletal muscle mass and/or fat-free mass and toxicity (10–15). Cespedes Feliciano et al. found that low muscle mass in the L3 area was associated with a discontinued course (odds ratio (OR) 2.34; 95 % confidence interval (CI) 1.04–5.24), postponed course (OR 2.24; 95 % CI 1.37–3.66) and dose reduction (OR 2.28; 95 % CI 1.19–4.36) among patients who received a combination of 5-fluorouracil, folinate and oxaliplatin (10). Neutropenia and thrombocytopenia occurred significantly more frequently in patients with low skeletal muscle mass than in patients with medium and high skeletal muscle mass. This association was not found for neuropathy. Jung et al. found that patients in the lowest psoas index quartile had a significantly higher degree of grade 3–4 neutropenia and dose-limiting toxicity compared with patients in the uppermost quartile. A standard deviation reduction in the psoas index was associated with increased probability of both grade 3–4 neutropenia (OR 1.36; 95 % CI 0.93–1.98) and total dose-limiting toxicity (OR 1.67; 95 % CI 1.13–2.46) (11). Prado et al. investigated the incidence of toxicity in treatment with 5-fluorouracil and observed that dose-limiting toxicity, postponed course or dose reduction was associated with a higher value of 5-fluorouracil per kg fat-free mass (18 mg/kg vs 16 mg/kg) (14). However, this was not associated with increased values of 5-fluorouracil per unit surface area or 5-fluorouracil per kg body weight. The authors identified a threshold value of 20 mg 5-fluorouracil per kg fat-free mass as a critical level for a sharp increase in the risk of developing toxicity (OR 16.75). A high dose of 5-fluorouracil per kg fat-free mass was more frequent among women than men in this study. A higher incidence of dose-limiting toxicity among women than among men was also observed by Ilich et al. (68 % vs 52 %) (13). The authors postulate that this may be due to women generally having a lower proportion of fat-free mass than men.
Williams et al. observed that patients with sarcopenia (low fat-free mass) had a higher degree of dose-limiting toxicity than patients without sarcopenia, although not significantly different (50 % vs 30 %) (15). Patients with adverse reactions tended towards a higher cumulative dose of 5-fluorouracil per kg of fat-free mass than patients without adverse reactions (105 mg/kg vs 93 mg/kg). In line with this, Ali et al. observed a threshold level of 3.55 mg oxaliplatin per kg fat-free mass (lean body mass) as a critical level for developing dose-limiting toxicity (12). Dose-limiting toxicity was detected in 38 % and 14 % of patients with high and low chemotherapy doses per kg fat-free mass, respectively.
Relationship between low BMI and toxicity
Two of the studies indicate an association between low BMI and dose-limiting chemotherapy toxicity (16, 17). Suga et al. found a higher incidence of oxaliplatin-induced vascular pain among patients with BMI < 22 kg/m2 compared with patients with a normal or high BMI (72 % vs 58 %; OR 0.48; 95 % CI 0.26–0.91) (17), while Park et al. found that patients with dose reduction (up to 60 %) had a significantly lower BMI than patients without dose reduction (23.1 kg/m2 vs 24.0 kg/m2) (16). However, Shahriari-Ahmadi et al. found a relationship between higher BMI (≥ 25 kg/m2) and increased prevalence of toxicity in the form of neuropathy (42 % and 87 % for those with low and normal or high BMI, respectively) (18).
Relationship between weight loss and toxicity
Aprile et al. investigated nutritional status in the form of weight loss and found that weight loss was associated with several types of adverse reactions (CTCAE grade ≥ 1) (19). Weight loss was particularly strongly associated with fatigue and anorexia, but also with fever and dehydration and to a lesser extent chills, neuropathy and anxiety. Weight loss was not associated with adverse reactions such as neutropenia and pain.
Discussion
The results of this literature review indicate that colorectal cancer patients with low muscle mass are more vulnerable to toxicity ensuing from therapy than patients with normal muscle mass. Several aetiological mechanisms have been proposed (9, 20).
Loss of musculoskeletal mass in cancer patients, including those with localised disease, may cause hormonal and metabolic changes as well as increased inflammation and oxidative stress. Increased systemic inflammation may result in sustained muscle loss and hence lower fat-free mass. Reduced fat-free mass leads to a smaller distribution volume, and this may lead to higher bioavailability of drugs that are distributed and metabolised in fat-free mass. Ali et al. and Prado et al. both found threshold levels for the quantity of chemotherapeutics administered per kg of fat-free mass above which the risk of dose-limiting toxicity rose markedly (12, 14). Similar results have also been described for other cancer types (9).
Changes in nutritional status may also affect pharmacokinetic factors, which may result in changes in absorption, distribution, metabolism and elimination of drugs (21). This in turn may affect the concentration of a drug in the blood, and hence vulnerability to toxic adverse reactions.
Factors such as sex, age, disease severity, diet, level of physical activity, compliance with treatment and various genetic polymorphisms may conceivably also contribute to explaining the relationship and are potential confounding variables that should be taken into account.
In five of the six studies that investigated the relationship between low muscle mass and chemotherapy toxicity, CT was used as measuring tool (10–12, 14, 15). Four of them segmented all musculature at L3 level (10, 12, 14, 15), while one only segmented psoas musculature at level L4 (11). It is reasonable to suppose that including several muscle groups at L3 level, and not only psoas musculature at L4 level, provides a better picture of the patient’s total musculoskeletal mass and fat-free mass.
In other studies, weight and height were used to estimate BMI (16–18) or weight loss (19). Although these are well established measures of nutritional status in clinical practice, they are suboptimal measures of the quantity or distribution of fat mass compared with fat-free mass, and changes in body composition may thus be camouflaged.
Strengths and weaknesses
Only four of the included studies have adjusted for potential confounding variables (10, 11, 16, 17). This means that it is not possible in most studies to exclude other possible causes of the observed association. In the following discussion, we present two examples of possible confounding factors: sex and disease severity.
Only two studies took account of sex, and both found that women experienced a significantly higher degree of toxicity than men (13, 14). The findings are supported by a meta-analysis of colorectal cancer patients (stages I–IV) who received bolus-based 5-fluorouracil in combination with folinate (22). The meta-analysis points to a possible pharmacokinetic explanation, in that the activity of the enzyme that metabolises 5-fluorouracil is generally lower in women than in men, but the documentation is inconsistent. The authors also ask whether there may be other pharmacokinetic or pharmacogenetic sex-related differences of significance.
The severity of the disease should also be taken into account, as this can affect the risk of malnutrition and toxicity. Four of the studies adjusted for cancer stage and/or functional status (10, 11, 13, 17). All found that the association between malnutrition and toxicity persisted. Studies that only included colorectal cancer patients with stage II or III cancer also found this association (10, 11, 14, 16).
All studies included in the review are historical cohort studies (23). As a general rule, one cannot draw any conclusions about causality in this type of study. On the whole, symptoms of malnutrition were measured before toxicity arose, which increases the probability that malnutrition increases the risk of toxic adverse reactions. The studies primarily used data from patient records to define exposure and outcome variables, which results in high study participation and hence a low risk of selection bias (23). A weakness of using patient record data is that we know little about how systematic the records are. In the studies that have looked at malnutrition in the form of BMI and weight loss, there is also some lack of clarity with respect to the time of recording weight and height, degree of weight loss, and whether account has been taken of any weight changes during chemotherapy (16–19).
The number of participants in the different studies varies substantially. Several have a small number of patients, which reduces the statistical robustness. Five of the studies also include patients with metastatic disease (12, 13, 15, 17, 19). In these studies, the whole population is analysed together, and we are therefore unable to say anything about the effects for patients with stage II–III cancer in isolation. However, the studies of Cespedes Feliciano et al. (10) and Jung et al. (11) included a large number of stage II–III colorectal cancer patients (553 and 229 patients, respectively). Both conclude that there is a significant association between low muscle mass and dose-reducing toxicity in connection with treatment with a combination of 5-fluorouracil, folinate and oxaliplatin.
In light of the limitations of observational studies, it is worth noting an ongoing phase 2 study in France among patients with localised colorectal cancer, where the effect of oxaliplatin-based chemotherapy scaled to patients’ fat-free mass is compared with the standard dosage regimen (24). The results of this study will hopefully yield better answers to whether dose-calculation based on body composition has a part to play in the chemotherapy of the future for this patient population.
Can the results be transferred to Norwegian conditions?
In Norway a combination course consisting of 5-fluorouracil, folinate and oxaliplatin has traditionally been administered as a bolus regimen to patients with stage II–III colorectal cancer. In recent years, however, there has been more of a shift to an infusion regimen, as discussed in this review (3). The results of this review show that there is a relationship between clinical signs of disease-related malnutrition and increased chemotherapy toxicity in patients with colorectal cancer. The results of studies that have investigated low fat-free mass appear particularly convincing. There is therefore reason to investigate whether knowledge of patients’ fat-free mass could contribute to more precise chemotherapy dosage for localised colorectal cancer. Clinical studies should investigate whether information about body composition should be incorporated in the current dose algorithm or used as guidance for therapy decision at administration. To ensure that alternative dosage regimens are not at the expense of therapeutic efficacy, it is important that the studies also include survival. The issue is probably also relevant for adjuvant therapy for other types of cancer.
Conclusion
At present, dosage of adjuvant chemotherapy for colorectal cancer patients is based on their surface area, a method that does not take account of individual body composition or pharmacokinetics. The results of this review indicate that there is a relationship between signs of disease-related malnutrition, particularly low fat-free mass, and the incidence of toxicity and hence modification of the therapy course. Abdominal CT scans are performed routinely for this patient population and can be used to estimate fat-free mass. New technology that enables a faster review of images than previously makes the information more readily accessible to health personnel. Future clinical studies should investigate the potential for basing chemotherapeutics dosage on body composition in addition to surface area as a step in therapy optimisation.
