A woman in her sixties sustained a cut to the patella and developed bursitis. The infection was treated and she was followed up as an outpatient. However, the bursitis recurred and the disease course was complicated by sepsis, disseminated intravascular coagulation and multiorgan failure.
A previously healthy woman in her sixties fell and hit her right knee, sustaining a 5 cm long cut over the patella. The wound was cleansed and sutured in Accident and Emergency (A&E), and tetanus prophylaxis was administered. One week later, the patient attended A&E again with clinical signs that the wound had become infected, namely pain in the knee, redness and swelling. She was referred to the emergency outpatient clinic at her local hospital for further assessment. At the hospital the wound was opened and debrided, and a sample was taken for culture. A sample of the wound exudate was also collected for bacteriological testing. X-ray of the knee revealed no fractures. Blood tests showed CRP 68 mg/l (reference range <5 mg/l) and leukocytes 9.2 ∙ 109/l (3.5–10 ∙ 109/l). The patient was afebrile. Antimicrobial treatment with dicloxacillin (1 g × 3) was started and the patient was sent home with an appointment for outpatient follow-up.
Common symptoms of wound infection are redness, swelling, pain and possibly exudate or pus. This type of infection can generally be managed with careful care of the wound, along with debridement of unclean wounds and drainage of any abscess (1). Antibiotics are often unnecessary.
Two days later, the patient was referred to Acute Admissions owing to vomiting and reduced general condition. Upon admission, she had blood pressure 107/56 mm Hg, a regular pulse of 83 beats/min, respiratory rate 22 breaths/min and temperature 37.5 °C. The knee now showed a prepatellar erythematous field of approximately 10 × 10 cm, with secretion of pus from a central wound. The knee was sore to palpation with reduced mobility owing to pain. Preliminary blood tests revealed CRP 129 mg/l and leukocytes 11.9 ∙ 109/l, but otherwise normal results. The sample collected at first contact showed no bacterial growth.
The current event was considered a failure of oral antibiotic therapy and treatment with cloxacillin (2 g × 4 intravenously) was started. Septic arthritis or cellulitis were considered possible differential diagnoses.
Septic arthritis is a bacterial infection of a synovial joint originating from an infected wound or bone, or from haematogenous spread in cases of bacteraemia. The most common causative agent is Staphylococcus aureus (> 50 %), but streptococci, gram-negative rods such as Haemophilus influenzae, anaerobic bacteria and Neisseria gonorrhoeae may also be present (2, 3). Most patients have a previous joint injury or prosthesis, but immunosuppressed patients, diabetics, those with HIV infection, and substance users are also at risk. Local clinical signs of wound infection, elevated infection parameters, bacterial growth in blood cultures, and positive culture or PCR of synovial fluid and biopsies lend support to the diagnosis. Septic arthritis should be assessed using arthroscopy and lavage, both for diagnostic purposes as well for treatment prior to the initiation of antibiotic therapy. Arthroscopy should be repeated if there is no improvement after three days of treatment (3).
The patient was hospitalised for eight days. During the admission, puncture of the knee joint was performed, which showed clear synovial fluid. This was sent to be cultured. At the same time, an incision was made in the prepatellar bursa, and slightly turbid wound exudate drained out. A sample of the exudate was also collected for culture. The wound cavity was flushed and a drain was inserted. Ultrasound of the lower extremity revealed no deep vein thrombosis. Measures of infection parameters showed only slow improvement during the ongoing cloxacillin treatment. Cloxacillin was therefore replaced with clindamycin, which produced clinical and biochemical improvement. Synovial fluid culture showed no growth, but PCR was not performed. CRP had fallen to 56 mg/l upon discharge. Bacterial prepatellar bursitis was diagnosed on the basis of clinical findings and symptoms. The patient was started on a course of clindamycin (Dalacin) and attended weekly outpatient appointments for three weeks. At these appointments, CRP continued to decrease. Growth of Pseudomonas aeruginosa and Serratia spp. was detected in the wound exudate from the prepatellar bursa. Resistance assays revealed sensitivity of both agents to oral ciprofloxacin. The patient’s treatment was therefore switched to ciprofloxacin. At the last check-up, the wound had healed and the patient was afebrile and able to put weight on the leg without significant pain.
Prepatellar bursitis is a fairly common condition and is usually aseptic. Precipitating factors include trauma and overloading of the joint. Inflammation leads to a marked increase in the fluid content of the affected bursa. Typical symptoms of bursitis are swelling, pain, redness and warmth. The diagnosis is often made on the basis of clinical examination, possibly supported by fluid aspiration and culture. Treatment consists of unloading the joint, pain relief, and cortisone injections if indicated. Antibiotic therapy and surgical drainage are often required in cases of infectious bursitis (4, 5).
Nineteen days after her last outpatient appointment, the patient was readmitted owing to three days of symptoms similar to those seen at her previous admission. She had blood pressure 136/72 mm Hg, regular pulse 78 beats/min, respiratory rate 18 breaths/min and temperature 36.9 °C. Preliminary blood tests showed CRP 70 mg/l, leukocytes 13.6 ∙ 109/l, creatinine 134 μmol/l (60–105 μmol/l) and glomerular filtration rate (GFR) 36 ml/min (> 60 ml/min). The patellar wound was now covered by a scab with no exudate, but the knee itself was red and swollen. Any palpation or movement of the knee caused severe pain.
The condition was interpreted as a recurrence of bacterial bursitis. Blood cultures were taken prior to initiating treatment with intravenous ciprofloxacin on the basis of the previous culture findings. It emerged that the patient had been taking ibuprofen for pain relief prior to her admission. She used no other nephrotoxic medications, and therefore non-steroidal anti-inflammatory drugs (NSAIDs) and infection were assumed to have contributed to her prerenal acute renal failure. Ibuprofen was discontinued and fluid therapy was initiated.
Two days after admission, the patient was showing signs of clinical improvement, but her CRP level increased to 311 mg/l, leukocytes to 21.4 ∙ 109/l and creatinine to 191 μmol/l. The patient developed increasing pain, swelling and erythema in her right knee. Infectious disease specialists suspected staphylococci as the causative agents, as these are the most common in cases of bacterial bursitis. The previously detected agents, P. aeruginosa and Serratia spp., were considered less likely, as there had been little response to adequate antibiotic therapy. The antibiotic therapy was switched to cloxacillin. The increasing renal failure was considered to be the result of antimicrobial therapy without dose adjustment, as well as dehydration and the use of NSAIDs.
Ultrasound of the right knee showed subcutaneous oedema ventrally in the joint, and fluid in the suprapatellar bursa. Serous fluid was drained from the prepatellar bursa and was sent for culture. Puncture of the knee joint was also performed, with no signs of purulent synovial fluid. The synovial fluid was again sent for culture, but PCR was not requested. The patient now had marked signs of infection despite surgical drainage and antibiotic therapy, and had increasing CRP and fever. There was no growth in the blood cultures, wound exudate from the prepatellar bursa or synovial fluid. Erysipelas and cellulitis were therefore now considered more likely diagnoses than recurrence of bacterial bursitis and septic arthritis.
Blood tests now showed CRP in the 300s, deterioration in renal function and a new-onset electrolyte imbalance. Ultrasound of the kidneys and urinary tract showed no signs of post-renal bladder outlet obstruction or hydronephrosis. That same evening, CK analysis was requested and revealed a value in excess of 5 000 U/l (reference range < 210 U/l for women).
Differential diagnoses at this point included an abscess or a deep soft tissue infection. Additional CT scans of the thorax, abdomen, pelvis and lower extremities were performed. The patient still had pain in the knee, although not in the thigh or calf, and increasing swelling of the right thigh was now also apparent. The antibiotic regimen was expanded to include clindamycin and piperacillin-tazobactam.
CT of the right lower extremity showed severe subcutaneous oedema, involving deeper structures such as the muscle and fascia (Figure 1). CT of the thorax and abdomen revealed phlegmon of the musculature of the abdominal wall. There were no radiological signs of gas production in deeper tissues. CT findings were consistent with a deep soft tissue infection, such as necrotising fasciitis. After a total of five days at the local hospital, the patient was transferred to the nearest central hospital with expertise in plastic surgery.
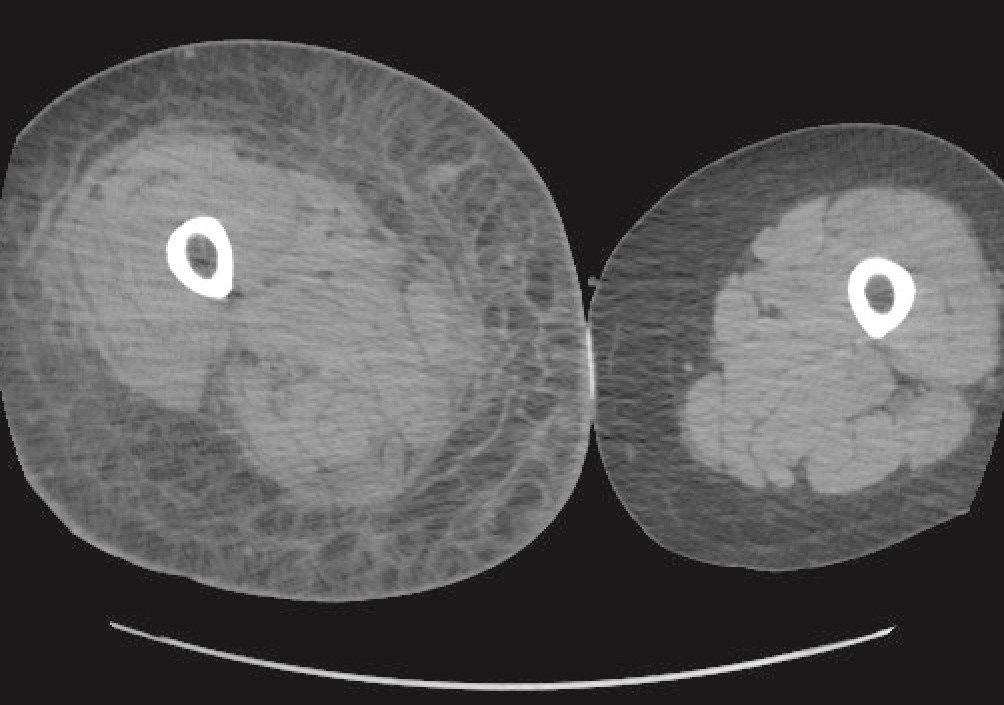
Figure 1 CT of lower extremity with subcutaneous oedema.
Necrotising soft tissue infections include fasciitis, myositis and cellulitis. The typical disease course entails fulminant tissue destruction with necrosis, systemic toxicity and high mortality (6). The infection can involve all layers of tissue. It can be difficult to distinguish necrotising fasciitis from myositis, as both skeletal muscle and fascia are affected in both conditions. However, necrotising fasciitis primarily involves fascia (7, 8).
Necrotising fasciitis is a rare disease. Its prevalence in Norway is unclear, but it has an annual incidence of about 1–5 cases per 100 000 population (9). It typically involves bacterial translocation along the muscle fascia, which has a poor blood supply. Infections can be divided into two types: type 1, which is a mixed infection with aerobic and anaerobic microbes (polymicrobial), and type 2, which is monomicrobial. Beta-haemolytic group A streptococci (GAS) are the most common aetiological agents in type 2 infections in Norway (6, 10), but S. aureus is also seen (7, 10). Type 2 infections can occur in previously healthy individuals without underlying disease. Group A streptococci are virulent and often toxin-producing microbes that can give rise to rapidly progressing necrosis in deeper tissues, with only modest involvement of the overlying skin and muscle. The most common locations of infection are the extremities, abdominal wall and perineum. Haematogenic spread may also occur, for example, after GAS tonsillitis (7, 10, 11).
Type 1 infections involve both aerobic and anaerobic microbes, usually gram-positive cocci (S. aureus, beta-haemolytic streptococci), gram-negative rods (Enterobacter spp., Escherichia coli, Klebsiella spp.) and anaerobes (Clostridium spp., Bacteroides spp., peptostreptococci) (6, 7, 11). Pseudomonas spp. and yeast fungi are uncommon (7, 12). Risk factors include advanced age, diabetes mellitus, peripheral vascular disease, pressure ulcers, haemorrhoids, rectal fissures and gynecological procedures. Certain microbes can produce gas, and therefore gas bubbles may occasionally be seen in the tissue (on CT scans). Both types of infections are characterised by a rapidly progressing course with systemic toxicity, often within 24 hours (12).
Erythema without a sharp demarcation from healthy skin, severe pain and localised tenderness in a (pre-)septic patient should raise suspicion of necrotising fasciitis. In 50 % of patients, palpation will reveal subcutaneous crepitation and oedema owing to the presence of gas-producing microbes (6, 11). Rapid surgical debridement is indicated and life-saving. Repeated surgical revisions and removal of new-onset necrosis are necessary for adequate infection control (11, 13).
On the day of transfer to the central hospital, the patient developed progressive multiorgan failure with hypotension, acidosis and increasing renal failure. GFR was 17 ml/min, CRP 255 mg/l and leukocytes >31 ∙ 109/l. The right thigh was now more than 20 cm larger than the left, and the patient had increasing pain in the thigh and contralateral trunk. Fasciotomy was therefore performed on the thigh, knee, calf and trunk. Clinical findings in the lower right extremity and trunk were consistent with necrotising fasciitis, with oedematous and fluid-filled adipose tissue, greyish areas of fascia, turbid fluid, atonic musculature with no response to diathermy, and areas of necrotic muscle (vastus lateralis). Aspirate was collected in blood culture bottles, and biopsies from muscle, fascia and adipose tissue were obtained for culture. Gram staining and direct microscopy of fascia specimens revealed gram-negative rods, but no growth occurred that could be used to identify the causative agents and therefore PCR of the biopsies was not performed. Antibiotic therapy was changed from clindamycin and piperacillin-tazobactam to double gram-negative coverage with meropenem, ciprofloxacin and clindamycin with a view to previous coverage of Pseudomonas spp. in particular, as well as anaerobic and gram-positive coverage. Amputation of the lower extremities was considered as a life-saving intervention, but was decided against as the infection had already spread to the trunk.
The day after the debridement, CRP decreased from 359 mg/l to 97 mg/l and leukocytes from 36 ∙ 109/l to 16.9 ∙ 109/l. Despite the biochemical improvement after the first partial debridement and the changes to antibiotic therapy, the patient’s multiorgan failure progressed. Debridement was performed four times in total. During the last debridement, healthier tissues without signs of necrosis were seen in the left lower extremity, and previously atonic muscle was now responding to diathermy, but the vastus lateralis muscle in the right lower extremity was still failing to contract and was considered to be necrotic. After three days at the central hospital, the patient was transferred to a university hospital for potential hyperbaric treatment. Prior to transfer, all blood cultures were negative. No growth occurred in any of the biopsies, with the exception of that from the right thigh, which showed growth of Candida albicans and Staphylococcus epidermidis. The latter was thought likely to reflect contamination from the skin. The tissue samples were not examined with bacterial or fungal PCR. Treatment with caspofungin (70 mg × 1) was attempted because of the detection of yeast fungi in the right thigh biopsy (Figure 2). Histological findings in the biopsies were consistent with necrotising fasciitis.
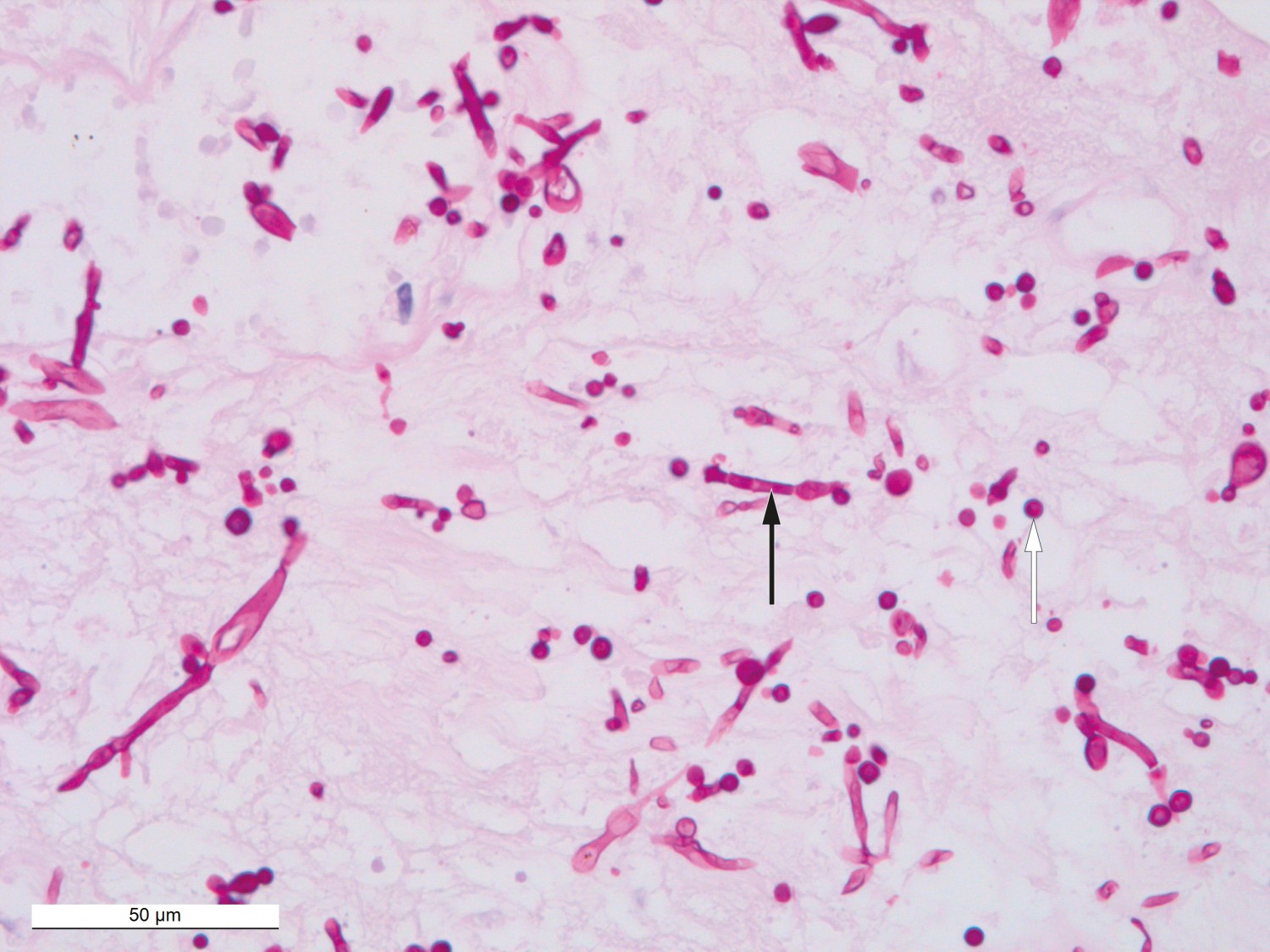
Figure 2 Histological PAS-stained section of tissue from the wound edge on the right knee showing positive results for fungal hyphae (black arrow) and spores (white arrow). Photograph taken through a microscope with x40 magnification.
At the university hospital, it was concluded that there were no other affected tissues in need of debridement at that point. CT thorax and pelvis showed cessation of circulation in the coeliac trunk artery and reduced circulation in the superior mesenteric artery. Despite all active interventions, the patient did not respond to treatment. She died and her body was sent for autopsy.
The autopsy revealed pronounced generalised oedema with pericardial fluid, ascites and pleural effusion, and sparse inflammatory changes in the right knee and calf. Immunohistochemistry confirmed the presence of yeast hyphae (Figure 3); however, post-mortem growth of the latter cannot be ruled out. Neither fungal elements nor inflammation were found in other organs, which supports the possibility of non-invasive candidiasis. Infection of the right knee was therefore regarded as the underlying cause of death, while clinical sepsis was considered the direct cause of death.
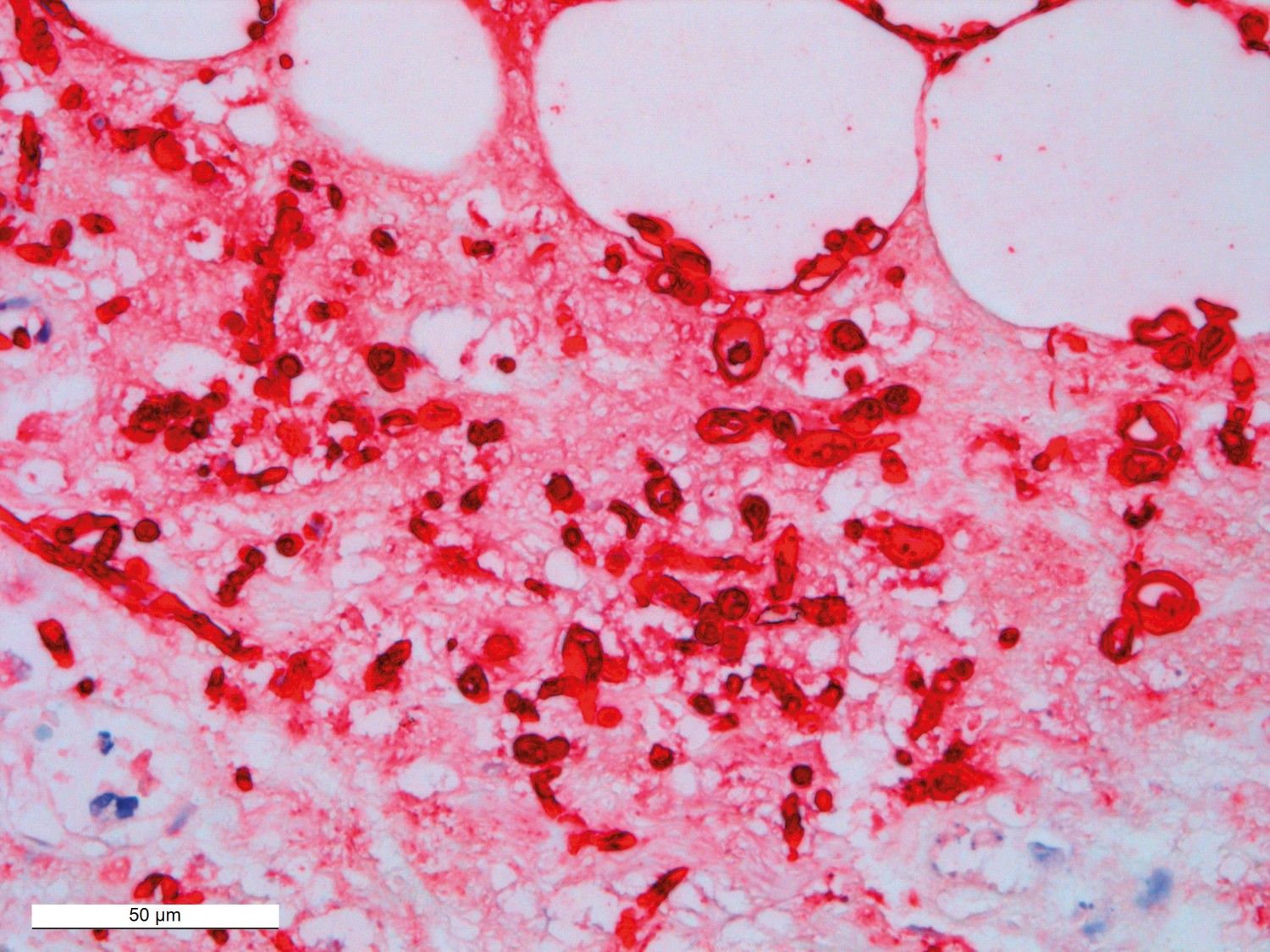
Figure 3 Immunohistochemistry was positive (red) for Candida.
Discussion
Necrotising soft tissue infections are characterised by an acute course that requires hospitalisation and prompt and effective treatment with debridement, antibiotics and intensive care. Norwegian guidelines for the antibiotic treatment of necrotising soft tissue infections with unknown aetiology recommend the use of benzylpenicillin (3 g × 4–6), clindamycin (600–900 mg × 3–4) and gentamicin (5–7 mg/kg × 1 intravenously). An alternative treatment regimen in cases of renal failure and GFR <30 is cefotaxime (2 g × 3) and clindamycin (600–900 mg × 3–4 intravenously) (13).
Necrotising fasciitis is a clinical diagnosis. In the current patient, the correct diagnosis was made too late as neither necrotising soft tissue infection nor necrotising fasciitis were initially suspected. Direct microscopy confirmed the presence of gram-negative rods, but growth of these was not seen. The absence of growth in multiple fascia biopsies, blood cultures and wound exudate specimens is probably due to the prolonged antibiotic treatment at the time of sampling. Unfortunately, PCR of bacterial DNA (16S DNA) and fungal DNA (18S DNA) were never requested. The causative agents responsible for the fasciitis were therefore never identified. The patient probably had a mixed infection (type 1 fasciitis) with gram-negative rods (Pseudomonas spp. and Serratia spp.) and anaerobic bacteria, without these being detectable. Fungi are opportunistic agents and are highly unusual in cases of necrotising fasciitis (12, 14, 15). The presence of yeast is probably related to the long-term antibiotic treatment that the patient received.
Necrotising soft tissue infections such as necrotising fasciitis can be challenging to diagnose. The most important point to remember is that necrotising fasciitis can occur in completely healthy individuals of all ages. It should be suspected in patients with signs of soft tissue infection, fever, haemodynamic changes and intractable pain that is inconsistent with clinical findings.