When assessing patients with fever and rash, the patient history, symptoms and the results of the clinical examination and supplementary tests must all be considered in combination. Here we present a patient in whom microbiological findings were crucial for the correct clinical diagnosis. A more thorough anamnesis could probably have led to more rapid clarification.
A previously healthy woman in her twenties was referred to the medical ward from the out-of-hours primary health care service after a 3-day history of fever, continuous headache, dizziness and nausea without vomiting. She had no neck stiffness or photophobia, but had a maculopapular, non-pruritic exanthema on her arms and on the palms of her hands.
The patient had taken paracetamol and ibuprofen, but to no effect. She had no respiratory or urinary tract symptoms, but reported having experienced a couple of episodes of chills in the days prior to admission to the hospital. Upon assessment in Acute Admissions, she was mentally alert with only slightly reduced general condition.
Upon admission, she had blood pressure of 118/82 mm Hg, a regular heart rate of 96 beats/min and a respiratory rate of 19 per minute. Oxygen saturation was 100 % in room air and she had an axillary temperature of 36.2 °C.
Examination revealed a maculopapular exanthema on the volar surface of the forearms and on both palms, as well as a small vesicle on the left thumb (Figure 1). There was no enanthema in the oral cavity or exanthema on the soles of the feet. Clinical examination showed no other abnormal findings. The cause of the symptoms was unclear, and the patient was admitted to the observation ward for further investigation.
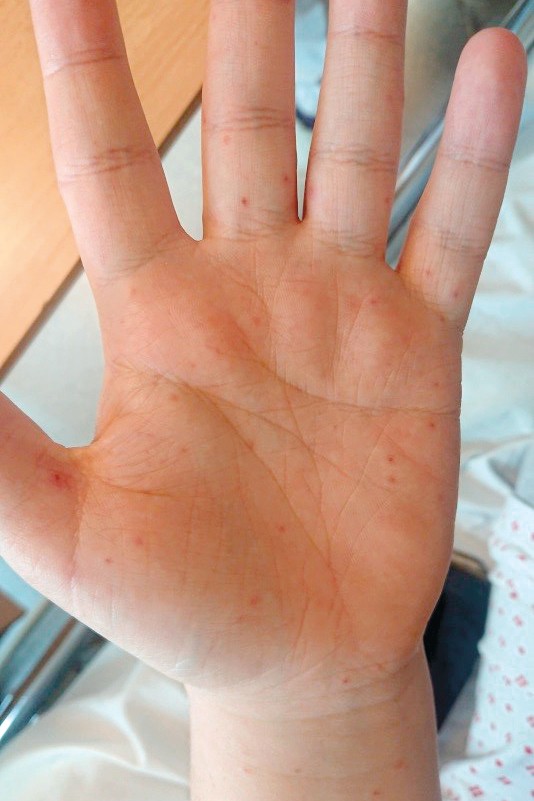
Figure 1 Exanthema on the patient’s left palm.
Maculopapular exanthema, which can also affect the palms and possibly the soles of the feet, can be caused by a variety of microbiological agents, drugs, malignancies and systemic diseases. Box 1 shows some of the key infectious agents and conditions that are typically responsible for such a rash in patients who become ill in Norway. In the event of a positive travel history, the list of potential causes may differ (1).
Box 1 Some of the most common viral and bacterial agents that cause exanthema affecting the palms and possibly the soles of the feet (1).
Viral infections
Coxsackievirus
Enterovirus
Epstein-Barr virus (EBV)
Cytomegalovirus (CMV)
Herpes simplex virus (HSV)
Varicella zoster virus (VZV)
Human herpesvirus 6 (HHV6)
Human herpesvirus 8 (HHV8)
Measles virus
Systemic bacterial infections
Meningococcal disease
Infective endocarditis
Syphilis
Toxic shock syndrome
Rat-bite fever
A drug rash should be considered a potential cause if the patient has taken or is taking any medications. Various haematological and oncological conditions can also cause exanthema affecting the palms and the soles of the feet (e.g. cutaneous T-cell lymphoma, mastocytosis and erythromelalgia). Other possible causes include underlying systemic inflammatory conditions (e.g. adult-onset Still’s disease, acute cutaneous lupus erythematosus, reactive arthritis and Kawasaki disease) (1).
The patient had not been taking any medications prior to becoming ill, so a drug-induced condition was considered unlikely. There were no clinical signs of a systemic bacterial infection, and therefore meningococcal disease and toxic shock syndrome were not regarded as differential diagnoses. The patient did not have a heart murmur, and the appearance of the exanthema was inconsistent with endocarditis. She had not been travelling abroad, and therefore the cause of the symptoms was not thought to be an imported disease.
Preliminary blood tests showed CRP 222 mg/l (reference <6 mg/l), leukocytes 13 ∙ 109/l (3.5–11.0 ∙ 109/l), neutrophilic granulocytes 9.2 ∙ 109/l (1.9–8.0 ∙ 109/l) and monocytes 1.32 ∙ 109/l (0.20–1.0 ∙ 109/l), but a normal differential count and no other abnormalities. Chest X-ray was normal. Urine dipstick testing performed in the out-of-hours primary health care service had shown 2+ for blood and 1+ for protein.
A nasopharyngeal specimen was obtained to test for respiratory viruses and ‘atypical bacterial agents’ (Mycoplasma pneumoniae and Chlamydia pneumoniae), along with a sample to perform serological testing for herpes simplex, varicella zoster and parvovirus. A sample from the small vesicle on the left thumb was sent for polymerase chain reaction (PCR) testing for herpes simplex virus types 1 and 2, varicella zoster virus and enterovirus. Serological testing for syphilis was not performed, as this was not considered a likely diagnosis based on the medical history and clinical presentation.
We suspected the patient’s symptoms had a viral origin and therefore chose not to initiate antibiotic treatment immediately.
The evening of her admission, the patient developed a fever of 38.2 °C, and two sets of blood cultures were taken. Over the course of the night her headache intensified and her temperature rose to 39.1 °C. The maculopapular exanthema worsened and could now be seen on both lower extremities and on parts of the trunk. She had mild tachycardia, with a heart rate of 106 beats/min, but otherwise stable vital signs. She was given 1 g paracetamol orally. Additional blood cultures were taken and she remained under observation, but antibiotic treatment was not initiated.
The patient was assessed and was judged not to have sepsis, but it was still unclear whether her symptoms had a bacterial aetiology. She was mentally alert, with no incipient neck stiffness and her respiratory rate was normal. The doctor on duty felt that delaying the use of antibiotics was still justified as the patient was under observation.
The following day, the patient’s general condition was good and she was afebrile. Her CRP level had decreased somewhat (from 222 mg/l to 194 mg/l) and her leukocyte count fallen slightly (from 13 ∙ 109/l to 11.8 ∙ 109/l).
A urine culture showed gram-positive mixed flora (1 000–10 000 CFU/ml). However, as the patient had no symptoms of a urinary tract infection, this was interpreted as contamination. Urine dipstick testing showed 1+ for protein and 1+ for glucose, but was negative for blood. Because the diagnosis was still unclear, the patient was transferred on day 2 from the observation ward to the infectious diseases department.
On day 3 she remained afebrile. A further spontaneous drop in CRP levels had occurred to 106 mg/l, and her leukocyte count had fallen to 9.4 ∙ 109/l. On arrival, the patient’s occupational history had not been noted in her medical records, and upon questioning, it now emerged that she worked as a veterinary nurse in a veterinary clinic. She denied having experienced anything out of the ordinary at work, including bites or scratches, in the time leading up to her illness. She was not asked at this point whether she had any pets or whether she had had contact with animals outside of work. The patient now considered herself almost restored back to normal health. Her rash had improved slightly and no new lesions had appeared in the last 24 hours. She was showing spontaneous clinical and biochemical improvement, and we believed a viral aetiology was most likely. We considered discharging the patient, but later that day a telephone message from the microbiology section reported the growth of gram-negative rods in three of four blood culture bottles. Intravenous treatment with cefotaxime 2 g × 3 was therefore initiated while waiting for the final identification of the bacterium.
There was no explanation in the medical records as to why treatment was not started, in line with the Norwegian national guidelines for antibiotic use, with gentamicin and benzylpenicillin. When the aim is to cover the most common gram-negative agents found in blood cultures in Norway, E. coli and Klebsiella spp., gentamicin is usually a better choice than cefotaxime as there is somewhat less resistance to the former (2). However, infection with the most common gram-negative rod bacteria would still not be able to explain the patient’s rash. One possibility is that because the focus of the infection and the agent were unclear, a drug with a gram-negative spectrum but better tissue penetration than gentamicin was chosen.
The following day, the microbe was identified as Streptobacillus moniliformis using MALDI-TOF MS (Matrix Assisted Laser Desorption Ionisation Time of Flight Mass Spectrometry). This instrument uses mass spectrometry to detect protein fragments belonging to microorganisms on the basis of their molecular mass and charge. The microbial proteins are fragmented and ionised, and the molecular mass of free ions is detected in a vacuum chamber. The spectral protein pattern of the microbe is then compared to established protein patterns from known microorganisms in a database (3).
S. moniliformis causes rat-bite fever, and in a new conversation with the patient it emerged that she had kept rats as pets for several years. Three or four days before the start of her symptoms, she had received a number of scratches to the chest from one of the rats. There was no swelling or irritation around these scratches and the lesions were not visible on inspection after her admission.
Samples taken on arrival for PCR and serological testing were negative for relevant viral agents.
Notably, the two extra blood cultures that were collected prior to treatment initiation, because of a fever peak of 39.1 °C the night the patient was hospitalised, showed no bacterial growth.
The patient was treated with intravenous cefotaxime 2 g × 3 for three days as an inpatient. On day 5 of her hospital stay, the resistance profile was available and the patient’s general condition was good. Treatment was therefore switched to intravenous ceftriaxone 1 g × 1 for a further four days after discharge.
Although there are no established breakpoints for S. moniliformis, this can be considered appropriate treatment based on the available literature and the resistance profile for the isolate in question (Table 1) (4–9).
Table 1
Resistance profile of Streptobacillus moniliformis. Minimum inhibitory concentration was determined using the agar gradient method (Etest) on Mueller-Hinton fastidious blood agar (MH-F agar).
|
Antibiotic
|
Minimum inhibitory concentration (MIC), mg/l
|
|
Cefotaxime
|
0.008
|
|
Ciprofloxacin
|
0.25
|
|
Erythromycin
|
1.0
|
|
Clindamycin
|
0.016
|
|
Penicillin G
|
0.064
|
|
Tetracycline
|
0.064
|
Ceftriaxone was chosen over benzylpenicillin primarily for logistical reasons, as the patient’s general condition was good and she did not need to be in hospital. Because she lived far away from the hospital, it would have been impractical for her to travel back and forth to receive intravenous benzylpenicillin four times a day. After a week, she was switched to peroral therapy, specifically phenoxymethylpenicillin 1 g × 4 for a further seven days.
There were no joint manifestations, no signs of serositis, and no respiratory or circulatory symptoms in the subsequent disease course. No additional follow-up was scheduled by the hospital following the completion of antibiotic treatment. During a telephone conversation many months later, she reported that she had not experienced any symptoms or signs of relapse, and that she continued to keep rats as pets.
Discussion
Rat-bite fever is thought to be caused by two different bacterial species associated with two different disease states. In the United States and Europe, the gram-negative bacterium Streptobacillus moniliformis gives rise to streptobacillosis, also known as Haverhill fever (4, 7) following an outbreak in 1926 in which the source of the infection was unpasteurised milk. Rat-bite fever caused by the spirochete Spirillum minus gives rise to spirillosis, also known as sodoku (from the Japanese so = rotte, doku = gift). This occurs in Asia but is very rare (4, 7–9).
Rat-bite fever can be transmitted via rat bites or via more superficial scratches, as in our patient. Infection can be transmitted via contact with secretions or excrement from colonised animals, and epidemics can occur through contamination of food and drink by rat faeces (4, 7, 9). Other animals such as mice, guinea pigs and squirrels, as well as carnivores such as cats, dogs, pigs and snakes that eat these rodents, can also be sources of infection (4, 8).
The risk of infection following a rat bite is estimated to be approximately 10 % (7). The bacteria that cause rat-bite fever are frequently present in the oropharyngeal flora of rodents. In the United States, it has been reported that 50–100 % of rats are colonised (4, 9). The time from bite to disease is 3–10 days in the case of streptobacillosis and 7–21 days for spirillosis (7).
The symptoms are non-specific and can resemble a number of other conditions. A detailed medical history is therefore very important for directing suspicion towards rat-bite fever. S. moniliformis can be difficult to culture (5, 8), but we observed growth in three out of four standard blood culture bottles after about three days of incubation. If rat-bite fever is suspected, good communication with the microbiology department can help to increase the chances of growth by enabling the incubation period to be extended if necessary, using enriched broth media and paying particular attention to any unusual findings upon microscopy of the Gram stain preparation (Figure 2).

Figure 2 Gram-stained preparation of Streptobacillus moniliformis showing the pleomorphic appearance of the bacterium. Note in particular the ‘string-of-pearls’-like morphology.
Streptobacillosis gives rise to a clinical picture characterised by fever, headache, nausea/vomiting, muscle and joint pain, as well as rash, including on the palms (7, 8).
Approximately 50 % of patients develop asymmetric polyarthritis or septic arthritis. Other complications that may occur are pneumonia, endocarditis, meningitis, abscesses, vasculitis, adrenal insufficiency and fulminant sepsis (6, 8).
Left untreated, the infection may resolve spontaneously, but it may also recur weeks or months later with febrile episodes of 3–4 days separated by fever-free periods (8). Our patient experienced no relapses or other complications after treatment. The condition can be fatal, particularly in infants, and a mortality rate as high as 13 % has been described in untreated cases (4, 8).
Spirillosis causes swelling around the bite, local lymph node swelling, rash, and muscle and joint pain. The disease may resolve quickly, but then recur on multiple subsequent occasions. In cases of spirillosis, the rat bite often heals after a few days. Between one and several weeks later, the site becomes swollen, inflamed and painful, and an ulcer may form (7, 8).
In the absence of proper antibiotic treatment, the usual course of spirillosis is recurrent episodes of fever lasting 3–4 days separated by fever-free intervals of 3–9 days. Spontaneous resolution usually occurs within 1–2 months, but cases have been described in which the febrile episodes continued for years (8). Complications including endocarditis, myocarditis, hepatitis, meningitis, anaemia and conjunctivitis have been reported (6, 8). Mortality in the pre-antibiotic era was 6–10 % (8).
Early antibiotic treatment is associated with a good prognosis. Both S. moniliformis and S. minus are sensitive to several antibiotics, including penicillin, which is the first-line therapy. In cases of penicillin allergy, tetracycline may be a suitable alternative (4, 8).
The recommended duration of treatment for uncomplicated cases is 14 days, usually in the form of 5–7 days of intravenous treatment followed by peroral treatment. In mild cases, peroral treatment alone may be considered (7–9).
A few cases of streptobacillosis have been reported in Norway, but no known cases of spirillosis. The condition is not notifiable, and therefore exact figures are difficult to obtain. Few articles have been published on the condition in Norway. In 1992, a case of septicaemia was described in a child (10), and in 2001 the case of a woman with arthritis and rat-bite fever was published (11). There are also a few older reports of single cases (12).
Diagnostics
A medical history containing details of any bites or scratches from rats or other rodents is essential. It is also important to remember that the bacterium can be transmitted through contact with secretions (including urine and faeces) from colonised animals or via contaminated food or water (5). A sample of pus or possibly blood is cultured in order to detect S. moniliformis. If these samples are negative but the disease is still suspected, a biopsy of the area around the bite may be considered in order to isolate the bacterium (5).
S. minus has never been successfully cultured in a laboratory, but the bacterium has been detected through direct microscopy (4, 8). S. minus is a short, thick, gram-negative helical rod. The bacterium has terminal flagella that can be seen in rapid movement under dark field microscopy.
The flagella can be stained with silver impregnation techniques, e.g. Fontana-Tribondeau staining (8).
S. moniliformis is a filamentous, pleomorphic, immobile gram-negative rod bacterium. Under the microscope, it may resemble a typical gram-negative rod bacterium that forms aggregates or chains. The bacterium may also have long threads that are occasionally fragmented (Figure 2). These threads may have a ‘string-of-pearls’-like morphology (moniliform bodies), the presence of which gives the bacterium a very characteristic appearance (4, 8).
S. moniliformis is microaerophilic and capnophilic. Its growth is inhibited by sodium polyanethole sulfonate (SPS), a substance that is often added to blood culture bottles. SPS acts as an anticoagulant and also inhibits antibacterial substances, including complement, in the blood. Nutrient agars with 10–20 % rabbit, horse or sheep serum or defibrinated blood will promote growth (4). On blood agars, the bacterium grows as small non-haemolytic cotton-like colonies after about three days of incubation. In broth media, characteristic airy ‘cotton balls’ are seen at the bottom of the broth after 2–10 days (Figure 3) (4, 5, 8).

Figure 3 Streptobacillus moniliformis in the growth medium BHI (brain-heart infusion) broth supplemented with 10 % horse serum. The picture was taken after four days of incubation at 35ᵒC in room air. The formation of so-called ‘cotton balls’ can be seen.
A thorough and complete medical history is required in order to correctly diagnose patients with a skin rash and fever. The history must include information about travel, sexual activity, medication use, contact with animals, and exposure to forests and water. In addition, the patient’s age, time of symptom onset, and the appearance, distribution and location of the rash may be helpful in the diagnosis.
Many people keep rats and other rodents as pets. It is important to be aware of rat-bite fever as a potential explanation for fever of unknown origin, as left untreated the disease can have a serious course. Interventions that could help prevent cases include reductions in the rat population, improvements to housing, secure waste disposal sites, safe water sources, and pasteurisation of milk. Laboratory workers should wear gloves when handling rats (7).
This case report illustrates the importance of a detailed medical history and close collaboration with the microbiology department to ensure correct diagnosis and treatment.
