International studies with 20 years of follow-up have shown that breast-conserving surgery and mastectomy have similar results with respect to breast cancer survival, and that breast-conserving surgery is in the process of replacing mastectomy (1–3). Studies have also shown that breast-conserving surgery results in greater patient satisfaction than simple mastectomy or mastectomy with breast reconstruction (4). The primary aim of breast-conserving surgery is to achieve a high survival rate with low local recurrence rates and good cosmetic results. Studies have shown that up to 20 % of the volume of the upper part of the breast can be removed and 5–10 % of the lower part without causing breast deformities. To achieve good cosmetic results with breast-conserving surgery in patients with large tumours, oncoplastic techniques must be used (4,5). Various surgical options are considered by an interdisciplinary team, which assesses tumour-related and therapeutic factors. The surgical method is then chosen in consultation with the patient (5).
A Norwegian study with data from 1986 to 2009 showed that the proportion of women with invasive breast cancer who received breast-conserving surgery increased from 5 % in 1986 to 51 % in 2005, before falling back to 42 % in 2009 (6). The reason for the variation is complex, and can probably be attributed to the interpretation of the results in a 2005 review by the Early Breast Cancer Trialists’ Collaborative Group, which indicated an increased incidence of local recurrences and higher breast-cancer mortality in connection with breast-conserving surgery than with mastectomy (7). A follow-up study in 2012 showed that there was no difference between the two surgical procedures when it came to overall survival (3). According to current national recommendations in Norway, tumour size does not contraindicate breast-conserving surgery provided there is no inflammatory breast cancer or extensive areas with precursors that cannot be removed, and the patient is not a bearer of a gene mutation (5). The target according to the breast cancer quality indicators of the European Society of Breast Cancer Specialists (EUSOMA) is for 85 % of patients with tumours of less than 30 mm to undergo breast-conserving surgery (2,8). Multifocality and age are not contraindications. Breast-conserving surgery is contingent on tumour-free margins and post-operative radiotherapy as part of the treatment. Local recurrence rates have then proved to be about 0.6 % per year (9), and ten-year relative survival to be 84 % (8).
Up to year 2000, 64 hospitals provided surgical treatment for breast cancer patients in Norway (10). The hospitals had and still have different structures, cultures and operation volumes. In this study we present the use of breast-conserving surgery as opposed to mastectomy as a surgical method over time and by treating hospital, age on diagnosis, detection method and histopathological tumour characteristics.
Material and method
The legal basis for use of the data in this registry study is the Norwegian Cancer Registry Regulations and Act (11,12). The project was recommended by the Data Protection Officer at Oslo University Hospital (19/15705).
The data were retrieved from the Cancer Registry of Norway’s databases, which are 99 % complete for breast cancer (13). We identified 47 004 women in Norway who received their first diagnosis of invasive breast cancer in the period 2003–2018 and were registered in the Cancer Registry’s databases (10). Parts of this period, 2003–2009, overlapped with an earlier study, thereby providing a fuller picture of the issue (6). We excluded women with distant metastases when diagnosed (n = 1 773) and those for whom no surgical method was recorded (n = 2 638), which left a study population of 42 593 women (Figure 1). Twenty-five hospitals with fewer than 100 registered operations in the period 2015–2018 were included in the group “Other hospitals” (Table 1). The diagnostic period was divided into four: 2003–06 (n = 9 793), 2007–10 (n = 9 713), 2011–14 (n = 11 044) and 2015–18 (n = 12 043). The patients were divided into five age groups: < 40, 40–49, 50–59 (screening age), 60–69 (screening age), 70–79 and > 79 when disease was detected. Breast cancers detected by screening and detected in intervals between screening (interval cancer) among women aged under 50 (n = 23) or older than 69 (n = 289) were included in the categories 50–59 years and 60–69 years in 2015–2018, in order to group together cases of breast cancer associated with screening.

Figure 1 The study population
Table 1
Number of patients who underwent breast cancer surgery and percentage with breast-conserving surgery in Norway in the period 2015–2018, by detection method and hospital. OUH = Oslo University Hospital UNN = University Hospital of North Norway
|
Hospital
|
Total
|
Breast cancer detected by screening
|
Interval cancer
|
Breast cancer detected outside BreastScreen Norway
|
|
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
|
All hospitals
|
12 043
|
65.9
|
4 353
|
83.7
|
1 361
|
66.1
|
6 329
|
53.6
|
|
Vestre Viken Trust, Drammen
|
1 107
|
78.4
|
436
|
91.3
|
106
|
82.1
|
565
|
67.8
|
|
Akershus University Hospital
|
1 046
|
65.9
|
318
|
83.3
|
136
|
64.0
|
592
|
56.9
|
|
OUH, Ullevål
|
945
|
64.7
|
339
|
85.0
|
91
|
67.0
|
515
|
50.9
|
|
Haukeland University Hospital
|
920
|
68.8
|
382
|
85.1
|
91
|
62.6
|
447
|
56.2
|
|
Innlandet Hospital Trust
|
828
|
63.6
|
337
|
84.0
|
78
|
60.3
|
413
|
47.7
|
|
St. Olavs Hospital
|
813
|
66.3
|
365
|
83.8
|
79
|
67.1
|
369
|
48.8
|
|
Stavanger University Hospital
|
739
|
69.7
|
348
|
80.5
|
86
|
72.1
|
305
|
56.7
|
|
Østfold Hospital Trust
|
592
|
73.1
|
212
|
89.2
|
53
|
86.8
|
327
|
60.6
|
|
UNN, Tromsø
|
546
|
72.9
|
208
|
91.3
|
69
|
82.6
|
269
|
56.1
|
|
Sørlandet Hospital Trust
|
539
|
67.2
|
215
|
87.9
|
54
|
75.9
|
270
|
48.9
|
|
Oslo University Hospital, Radiumhospitalet
|
512
|
38.5
|
76
|
56.6
|
85
|
38.8
|
351
|
34.5
|
|
Vestfold Hospital Trust
|
414
|
85.0
|
177
|
93.2
|
44
|
84.1
|
193
|
77.7
|
|
Telemark Hospital Trust
|
405
|
68.1
|
144
|
81.3
|
42
|
76.2
|
219
|
58.0
|
|
Nordland Hospital Trust, Bodø
|
394
|
71.8
|
158
|
86.1
|
44
|
65.9
|
192
|
61.5
|
|
Møre og Romsdal Hospital Trust, Ålesund Hospital
|
393
|
76.6
|
187
|
93.6
|
45
|
80.0
|
161
|
55.9
|
|
Førde Hospital Trust
|
173
|
67.6
|
60
|
91.7
|
26
|
65.4
|
87
|
51.7
|
|
Fonna Hospital Trust, Haugesund Hospital1
|
172
|
40.7
|
-
|
-
|
34
|
44.1
|
136
|
39.7
|
|
Møre og Romsdal Hospital Trust, Molde Hospital
|
162
|
74.7
|
6
|
66.7
|
22
|
90.9
|
134
|
72.4
|
|
Nord-Trøndelag Health Trust, Levanger Hospital
|
108
|
76.9
|
13
|
76.9
|
15
|
86.7
|
80
|
75.0
|
|
Other hospitals2
|
124
|
40.2
|
7
|
71.4
|
17
|
58.8
|
100
|
34.7
|
|
Information not available
|
1 111
|
46.2
|
363
|
60.6
|
144
|
41.0
|
604
|
38.7
|
The surgical methods most recently reported to the Cancer Registry of Norway were used to divide the study population into two categories: breast-conserving surgery and mastectomy. Breast cancer detected by screening was defined as breast cancer diagnosed after findings in a screening examination, and interval cancer as breast cancer diagnosed after a negative result from a screening examination but before the next planned screening or within two years of the last screening for women in the age group 50–69 years. Breast cancer outside the screening programme covered women who had never received an offer to take part in the Mammography Programme, had never taken part, or had not taken part for the last two years (previous screening round).
We performed descriptive analyses which are presented as absolute figures and percentages of chosen surgical methods over time for the whole period, by age and by detection method. In addition we describe the current situation (2015–2018) in terms of treating hospital, age, detection method, tumour diameter and lymph node status. The statistical software package Stata MP version 16.0 (StataCorp, Texas, USA) was used for analyses.
Results
We found that 23 661/42 593 (55.6 %) of the included women with breast cancer detected in the period 2003–2018 received breast-conserving surgery (Figure 1). The proportion increased from 1 189/2 423 (49.1 %) in 2003 to 2 070/2 958 (70.0 %) in 2018, and was highest for women whose breast cancer had been detected by screening (Figure 2). In the period 2015–2018, the proportion was highest for breast cancer detected by screening at Møre og Romsdal Health Trust, Ålesund Hospital, with 175/187 (93.6 %), and lowest for breast cancer outside the screening programme at Oslo University Hospital, Radiumhospitalet, with 121/351 (34.5 %) (Table 1). The percentage of women who underwent breast-conserving surgery was highest among women in the screening age groups (50–59 and 60–69) and lowest amongst the oldest women (> 79) (Figure 3).
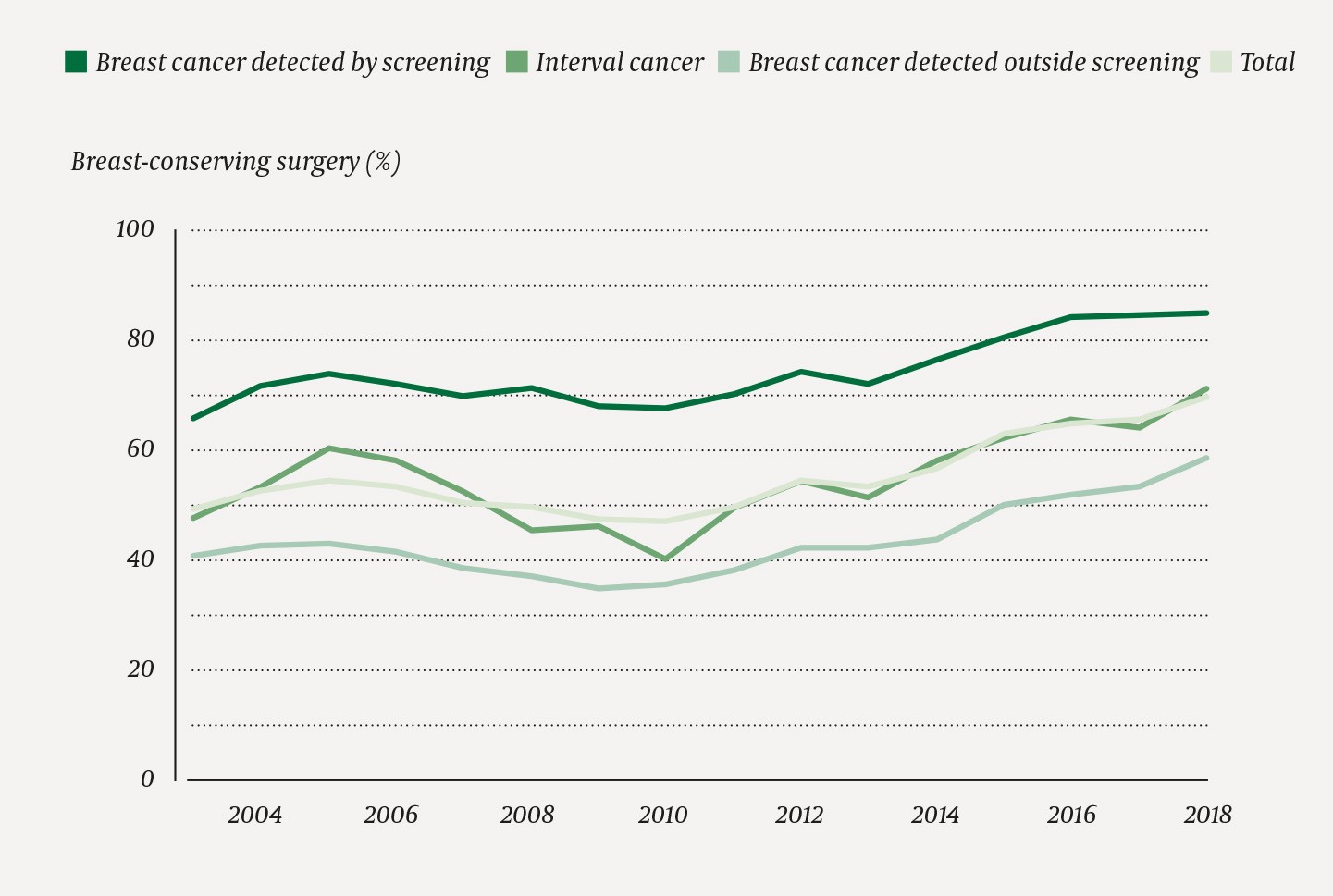
Figure 2 Percentage of women undergoing breast-conserving surgery of all women operated upon for breast cancer in Norway in the period 2003–2018 (n = 42 593) by detection method and calendar year
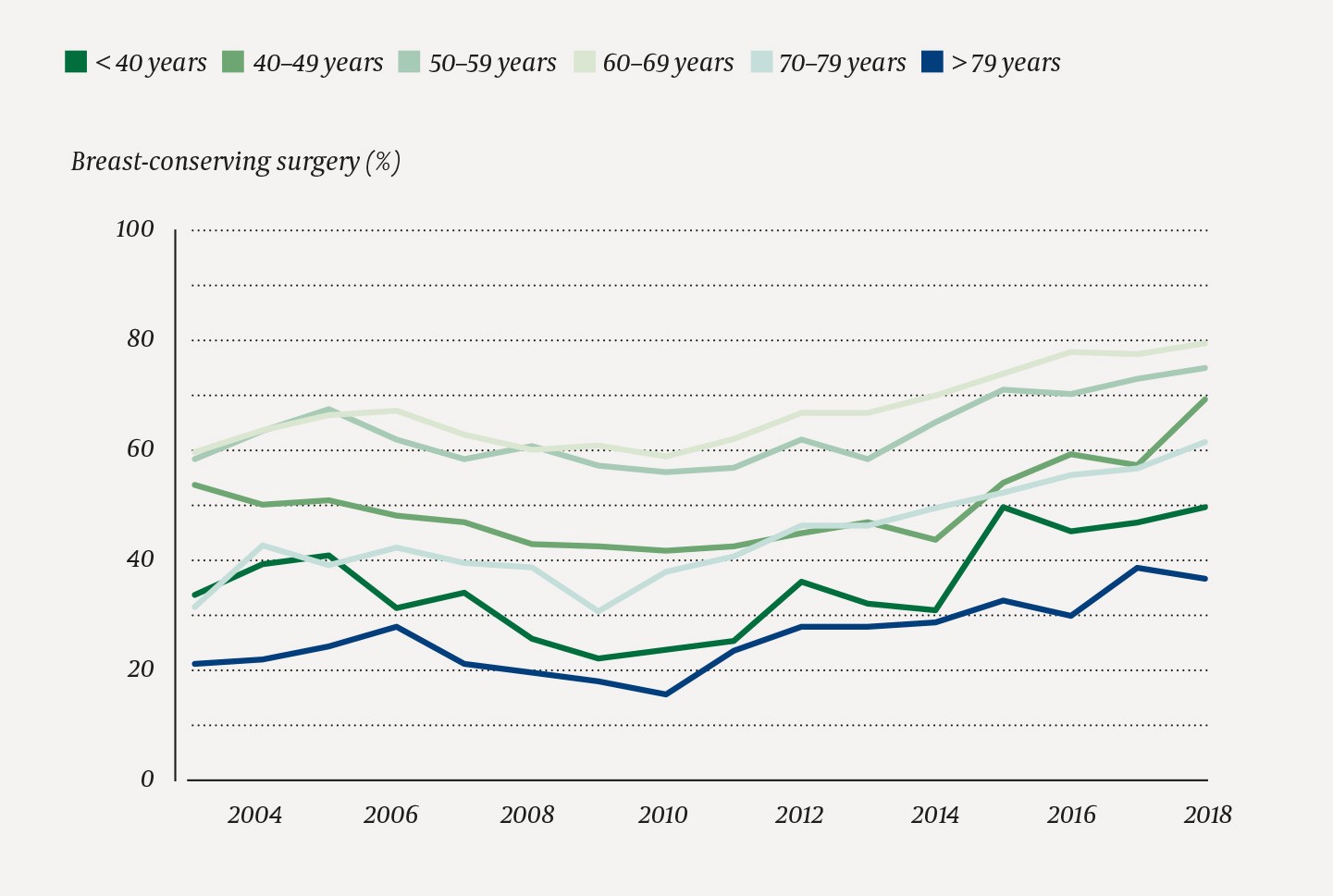
Figure 3 Percentage of women undergoing breast-conserving surgery of all women operated upon for breast cancer in Norway in the period 2003–2018 (n = 42 593) by age at time of diagnosis and calendar year
Breast-conserving surgery was used most frequently on women with breast cancer detected by screening and a tumour diameter of 10 mm or less, and the proportion in this group was 1 419/1 573 (90.2 %) (Table 2). We found that 7 322/10 077 (72.7 %) of women with tumours with a diameter of 30 mm or less had undergone breast-conserving surgery in the period 2015–2018. If tumour diameter is not taken into account, the proportion of breast-conserving surgery was lowest for women diagnosed with breast cancer outside the screening programme and who had tumours with metastasis to lymph nodes (861/2 066, 41.7 %).
Table 2
Number of patients operated upon for breast cancer and percentage with breast-conserving surgery in Norway in the period 2015–2018 by detection method, age, tumour diameter and lymph node status
|
|
Total
(n = 12 043)
|
Breast cancer detected by screening
(n = 4 353)
|
Interval cancer
(n = 1 351)
|
Breast cancer detected outside BreastScreen Norway (n = 6 329)
|
|
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
Breast cancer surgery (N)
|
Breast-conserving surgery (%)
|
|
Total
|
12 043
|
65.9
|
4 353
|
83.7
|
1,361
|
66.1
|
6 329
|
53.6
|
|
Age
|
|
|
|
|
|
|
|
|
|
|
Age
|
|
|
|
|
|
|
|
|
|
|
< 40 years
|
515
|
48.2
|
-
|
-
|
-
|
-
|
515
|
48.2
|
|
|
40–49 years
|
1 808
|
59.9
|
-
|
-
|
-
|
-
|
1 808
|
59.9
|
|
|
50–59 years1
|
3 108
|
72.8
|
1 800
|
79.7
|
581
|
67.5
|
727
|
59.8
|
|
|
60–69 years2
|
3 870
|
77.5
|
2 553
|
86.6
|
780
|
65.0
|
537
|
52.5
|
|
|
70–79 years
|
1 762
|
57.0
|
-
|
-
|
-
|
-
|
1 762
|
57.0
|
|
|
> 79 years
|
980
|
35.0
|
-
|
-
|
-
|
-
|
637
|
35.0
|
|
Tumour diameter
|
|
|
|
|
|
|
|
|
|
|
≤ 10 mm
|
2 751
|
79.9
|
1 573
|
90.2
|
249
|
72.7
|
929
|
64.5
|
|
|
11–20 mm
|
4 841
|
74.9
|
1 911
|
87.2
|
542
|
76.0
|
2 388
|
64.8
|
|
|
21–30 mm
|
2 485
|
60.3
|
556
|
75.7
|
315
|
69.8
|
1 614
|
53.2
|
|
|
31–40 mm
|
862
|
42.1
|
159
|
58.5
|
100
|
44.0
|
603
|
37.5
|
|
|
41–50 mm
|
305
|
24.6
|
47
|
34.0
|
39
|
30.8
|
219
|
21.5
|
|
|
> 50 cm
|
390
|
11.0
|
58
|
17.2
|
54
|
16.7
|
278
|
8.6
|
|
|
Information not available
|
409
|
33.0
|
49
|
40.8
|
62
|
33.9
|
298
|
31.5
|
|
Lymph node status
|
|
|
|
|
|
|
|
|
|
|
Negative
|
8 426
|
72.8
|
3 514
|
87.7
|
915
|
71.9
|
3 997
|
59.8
|
|
|
Positive
|
3 288
|
49.3
|
792
|
66.3
|
430
|
54.4
|
2 066
|
41.7
|
|
|
Information not available
|
329
|
57.1
|
47
|
80.9
|
16
|
43.8
|
266
|
53.8
|
Discussion
We found that the percentage of women who underwent breast-conserving surgery increased from 49.1 % in 2003 to 70.0 % in 2018, and that breast-conserving surgery was more usual for women with cancer detected by screening than for women with interval cancer or breast cancer detected outside BreastScreen Norway. The use of breast-conserving surgery varied across hospitals. We found that women with small tumours without spreading to axillary lymph nodes were more often able to keep their breast than women with large tumours and/or metastasis to the axilla.
Despite evidence-based recommendations regarding surgical method (5), practice varied across hospitals. The distance to hospitals that offer radiotherapy may conceivably have a bearing on the choice of procedure. Since 2014, hypofractionated radiotherapy has been most widely carried out, with 15 treatments compared with 25 previously (8). This may make it simpler to perform a breast-conserving procedure with subsequent radiotherapy.
The patient base and volume varied across hospitals. Treatment of locally advanced disease with systemic pre-surgical therapy is centralised to the university hospitals. Up until recent years, mastectomy has been the recommended procedure for this patient population. This may explain some of the difference between university hospitals and other hospitals. Different procedures may also be used in cases of multifocality, even though this has not been a contraindication for breast-conserving surgery in recent years. There are also probably different attitudes to and different expertise in and experience with oncoplastic techniques at the different hospitals. A new toolkit enables patients to be involved in making decisions about breast cancer surgery (14), but the individual surgeon probably still exerts influence on the choice of procedure. Recent annual reports from the National Quality Register for Breast Cancer may also have enhanced the trend of increasing proportions of breast-conserving surgery by revealing the differences across hospitals (8).
Information to patients is important, and may be influenced by the knowledge level, attitudes and technical skills of the person providing the information. An American study from 2006 demonstrated that patients who are informed by a radiation oncologist or breast surgeon more often choose breast-conserving surgery than those who are informed by a general surgeon (15).
If the tumour diameter is less than 20 mm, the breast volume is often sufficient for the tumour to be removed without oncoplastic techniques. Women who participate in BreastScreen Norway have a greater likelihood of having small tumours detected than those who do not participate. This forms a basis for less radical surgery. If the tumour diameter is over 30 mm, the use of breast-conserving surgery is reduced for all detection methods because of increased risk of breast deformities. Oncoplastic techniques may be used in many cases, but in the case of deficient experience and qualifications, mastectomy may be the only real option. Results from the National Quality Register for Breast Cancer may indicate that not all hospitals offer this type of surgery to their patients (8).
In 2016 the recommendations of the National Breast Cancer Treatment Programme were changed so that women with HER2-positive or stage 2 triple negative breast cancer were recommended systemic pre-surgical therapy (5). This has increased the possibility of performing breast-conserving surgery in the event of a good response to pre-surgical therapy. There is now international debate as to whether the time for systemic therapy should be prior to surgery in all cases where it is known at the time of diagnosis that systemic treatment is to be administered (16). This will require interdisciplinary teams of oncologists, radiologists, surgeons and pathologists, which not all hospitals that operate upon breast cancer patients in Norway have today.
We see an increase in the proportion of breast-conserving surgery among women under the age of 50. Studies have shown that it is safe also for women aged under 40 to keep their breast (17,18). In the US, an increased rate of mastectomies, combined with contralateral prophylactic mastectomy, is seen in younger patients (19). This is not recommended and has not been observed in our study.
We find the greatest change in surgical method among women aged over 70. In 2009, only 30 % of women aged 70–79 underwent breast-conserving surgery, compared with 62 % in 2018. Hypofractionated radiotherapy, introduced in 2010, is less stressful and may be of importance. Studies indicate that it may be safe to omit radiotherapy in breast-conserving surgery in older low-risk patients who receive endocrine therapy (20,21). This may further increase the proportion of breast-conserving surgery in this patient population.
The diameter of the tumour and spread to lymph nodes influence the choice of procedure. Tumour size does not contraindicate breast-conserving surgery unless tumour-free margins are not achieved, there is inflammatory breast cancer or there are extensive areas with precursors that cannot be removed (5). The requirement of free margins was changed during the study period, from at least 3 mm in 2003 to tumour-free margins in 2018 (5). This has a bearing on the size of the resected tissue, so that the conditions are often now more propitious for breast-conserving surgery.
As this is a register study, we do not know anything about the reason for performing mastectomies. Another weakness of the study is that there may be under-reporting of subsequent surgical treatment in connection with a breast-conserving operation. Re-operation does not represent a new case of cancer, and is not subject to the duty to report, and may therefore lead to under-reporting of the number of mastectomies. However, figures from the National Quality Register for Breast Cancer show that 91.8 % of breast cancer patients who underwent surgery in 2018 only had one procedure for a primary tumour (8). Nor do we have any information about gene mutation status, multifocality or the extension of precursors around the tumours. The strength of the study is that the Cancer Registry of Norway’s database is 99 % complete with respect to breast cancer, and 95 % complete with respect to surgical treatment (8,13).
Summary
We found that a larger proportion of women in whom breast cancer is detected receive breast-conserving surgery today than in the past. The proportion of breast-conserving procedures varied across hospitals, partly because of the patient base. Efforts should be made to comply with the national recommendations, so that women with breast cancer have the same options wherever in Norway they undergo surgery.
