Thrombocytopaenia is defined as a platelet count of < 150 · 109/L and occurs frequently in the critically ill. The condition arises as a result of the reduced production or increased destruction or consumption of platelets. Thrombocytopaenia in critically ill patients is often the result of multiple pathophysiological mechanisms, and these must be identified if the prognosis is to be determined and appropriate treatment provided.
Thrombocytopaenia occurs frequently in the critically ill, and often has several underlying causes. This article provides an overview of thrombocytopaenia in critically ill patients, including its pathophysiological mechanisms, course, assessment and treatment. The article is based on literature identified through a non-systematic search of the PubMed database and the authors’ own clinical experience.
Prevalence and definition
The incidence and prevalence of thrombocytopaenia vary depending on the study population and hospital department, and on the definition of thrombocytopaenia used (1). In a recent clinical study of patients in intensive care and observation units, thrombocytopaenia was defined as mild (100–149 · 109/L), moderate (51–99 · 109/L) or severe (<50 · 109/L), with reported incidences of 15.3 %, 5.1 %, and 1.6 %, respectively (2). The incidence of new-onset thrombocytopaenia among intensive care patients varies from 14 % to 44 % (1, 3). More than half of those who spend more than two weeks in intensive care develop thrombocytopaenia, which is associated with high mortality (4).
Pathophysiological mechanisms
Platelets are produced in the bone marrow from megakaryocytes, have a lifespan of 8–10 days and are broken down mainly in the spleen (5). They are essential for haemostasis, and also contribute to angiogenesis and innate immunity (5). The causes of thrombocytopaenia can be divided into three main categories: reduced production, increased destruction, and increased consumption of platelets. A single patient may often exhibit more than one of these pathophysiological mechanisms simultaneously (Figure 1). Platelet dysfunction is relatively common in critically ill patients and may result from uraemia, liver failure, or the use of medications or a cardiopulmonary bypass machine (6).
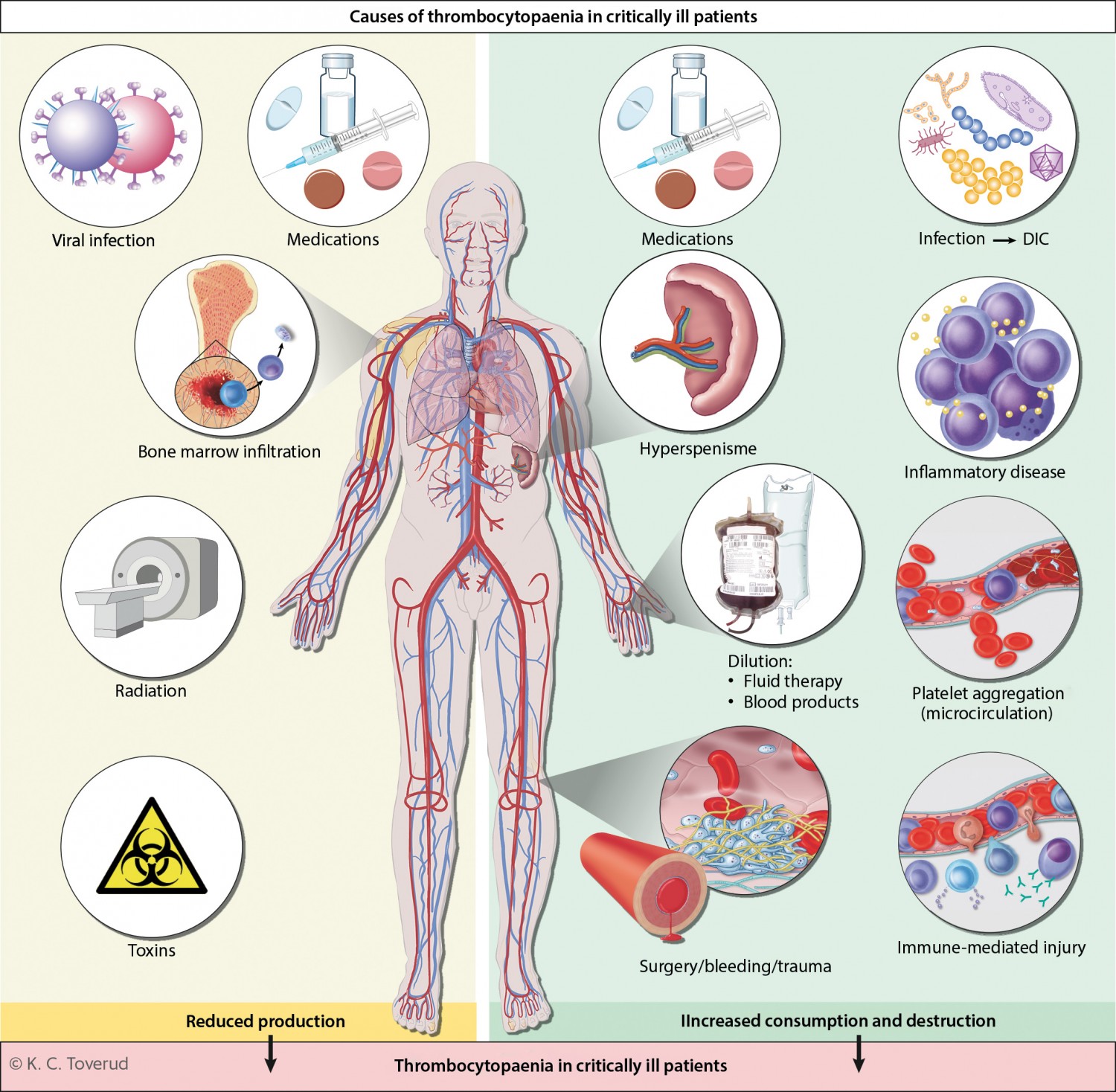
Figure 1 Thrombocytopaenia is common in critically ill patients, and is often the result of several different pathophysiological mechanisms. The causes can be divided into three main categories: reduced platelet production (bone marrow disease, use of chemotherapy or radiotherapy, acute and chronic viral infections, and poisonings); increased platelet consumption (physiological activation of platelets during surgery/trauma/bleeding, pathological activation of platelets as in cases of disseminated intravascular coagulation (DIC), which is most often triggered by severe infection/sepsis) and increased platelet destruction (hypersplenism, immunologically mediated drug hypersensitivity reactions and formation of autoantibodies, inflammatory diseases, such as haemophagocytic lymphohistiocytosis, and platelet aggregation in the microcirculation as in cases of thrombotic microangiopathy). In addition, thrombocytopaenia may occur as a result of a dilution effect upon massive transfusion of fluid or blood products.
Reduced production
It is unclear to what extent reduced platelet production contributes to thrombocytopaenia in critically ill patients. Individuals who have undergone cytotoxic chemotherapy show reduced platelet production, as do those with bone marrow disease or acute alcohol toxicity. Both acute and chronic viral infections, including hepatitis C, cytomegalovirus, Epstein-Barr virus and parvovirus B19, can inhibit platelet production (7). Thrombocytopaenia has also been described in patients with severe COVID-19 (8).
Increased consumption
Platelets can be activated by leukocytes, the complement system, coagulation factors, microbes and tissue damage. They also play a key role in the immune response (9). Thrombin-mediated activation of platelets can be physiological, for example in response to major surgery, trauma or blood loss. Pathological activation of platelets occurs in cases of disseminated intravascular coagulation (DIC) (10), which may be triggered by sepsis or malignancy, for example (11). Activated platelets are rapidly removed from the circulation, resulting in thrombocytopaenia. Patients receiving treatment with extracorporeal membrane oxygenation also show increased platelet consumption and often develop thrombocytopaenia.
Increased destruction
More than 50 % of cases of thrombocytopaenia in the critically ill are the result of sepsis or severe infection (12). Both antiplatelet drugs and antibiotics have been described as causing immune-mediated adverse reactions that may result in thrombocytopenia (13). Together these account for about 15 % of cases of thrombocytopaenia in intensive care units (12). The formation of antibodies can also lead to thrombocytopaenia. The most common example of this is the formation of antibodies against the heparin-platelet factor 4 (PF4) complex; these antibodies cause heparin-induced thrombocytopaenia (HIT) when heparins are used as anticoagulants. However, this is relatively rare and is reported in < 5 % of patients with thrombocytopaenia in intensive care units (14).
Autoantibodies in cases of immune thrombocytopaenia (ITP) are an uncommon cause of new-onset thrombocytopaenia in the critically ill, but have been reported previously (15). Alloantibodies, which can give rise to post-transfusion purpura, are extremely rare. Thrombotic thrombocytopenic purpura (TTP) may be congenital or secondarily acquired (16). Haemophagocytic lymphohistiocytosis (HLH) is an extremely serious hyperinflammatory condition with lymphocyte and macrophage activation, in which thrombocytopaenia may be part of the clinical picture (17, 18).
Assessment, diagnosis and clinical course
The underlying cause of thrombocytopaenia can often be identified by taking a thorough medical history and monitoring the clinical course, and by performing targeted laboratory diagnostic tests. In about 25 % of critically ill patients with thrombocytopaenia, the condition has more than one underlying cause (12) (Figure 1). It is essential to obtain an overview of the patient’s past medical history, comorbidities and medications and then view the patient’s current clinical status in light of this information. One must try to determine whether thrombocytopaenia is the result of the current critical illness and associated organ dysfunction, or whether it is part of a pre-existing underlying condition, such as serious malignant haematological disease.
The absolute platelet count should also be considered, along with the development and course of the thrombocytopaenia. Specific patterns will often become apparent which may reveal the underlying cause (Table 1). In general, thrombocytopaenia will be more severe (< 50 · 109/L) if a chronic underlying cause is accompanied by additional new-onset aetiological factors; platelet transfusion may then fail to increase the platelet count. Because of the high turnover of platelets, low platelet counts in cases of critical illness are likely to pose a lower haemostatic risk than thrombocytopaenia caused by bone marrow failure (19).
Table 1
Typical clinical courses of thrombocytopaenia in critically ill patients and possible underlying causes (4).
|
Clinical course
|
Probable cause
|
|
Platelet count low and remains low
|
Bone marrow failure
Hypersplenism
|
|
Platelet count rapidly declines, but then quickly normalises
|
Surgery
Use of cardiopulmonary bypass machine
Massive transfusions without platelets
|
|
Gradual decline and normalisation of platelet count as clinical condition improves
|
Sepsis
Pancreatitis
Inflammatory conditions
|
|
Platelet count falls and remains low despite clinical improvement in the patient
|
Drug-induced thrombocytopaenia
|
|
Platelet count falls and remains low in patients with persistent organ failure
|
Sepsis
Disseminated intravascular coagulation (DIC)
Circulatory failure
|
It is also important to be aware of other factors that may contribute to a low platelet count. Examples include pseudothrombocytopaenia due to EDTA-induced aggregation of platelets, haemodilution due to fluid infusion, massive transfusions of other blood components without platelets, or accumulation of platelets in the spleen in cases of splenomegaly or liver disease with portal hypertension. Patients with thrombocytopaenia who develop thromboembolic disease should be tested for procoagulatory conditions.
Treatment
In critically ill patients, thrombocytopaenia or a rapid fall in platelet count are markers of a poor prognosis and increased mortality risk. The patient’s underlying condition must be treated. Infection control and organ support therapy must be prioritised if the thrombocytopaenia is caused by sepsis (20), whereas haemostasis and blood transfusions are appropriate if the thrombocytopaenia is the result of massive bleeding.
Providing the correct treatment is dependent on identification of the underlying causes. Platelet transfusion may be the correct approach in patients with conditions that reduce the production or increase the consumption/destruction of platelets, but may potentially be harmful in conditions where there is increased intravascular activation of platelets, such as heparin-induced thrombocytopaenia, thrombotic thrombocytopaenic purpura or prothrombotic disseminated intravascular coagulation (21, 22). Platelet transfusion can also increase the risk of nosocomial infections and transfusion-related (acute) lung injuries (23–25). These factors justify a conservative approach to the use of platelet transfusions, with more emphasis placed instead on treating the underlying condition.
Therapeutic transfusion
To avoid severe thrombocytopaenia and haemodilution of coagulation factors, severe bleeding should be treated with a balanced transfusion of red cells, plasma and platelets in a 1: 1: 1 or 1: 2: 1 ratio. The optimal proportion of platelets is unclear (26), and in some institutions whole blood is used for transfusion in cases of severe bleeding (27). According to international guidelines, platelet transfusion should be considered for critically ill patients with bleeding and a platelet count of < 50 · 109/L, or in whom reduced platelet function is suspected/confirmed (20, 25).
Prophylactic transfusion
The most common reason for giving platelet transfusions to critically ill patients is to prevent bleeding. Clinical practice varies considerably, but international guidelines recommend platelet transfusion if the platelet count is < 10 · 109/L (20, 25). Transfusion of platelets is estimated to increase platelet count by about 15 · 109/L per transfusion unit (19), although this can vary to a relatively large degree.
Procedure-related transfusion
Platelet transfusion is recommended to minimise the risk of bleeding during certain procedures, for example during implantation of a central venous catheter in patients with a platelet count of < 20 · 109/L (25, 28). There are likewise guidelines and recommendations for the absolute platelet count in patients undergoing lumbar puncture or epidural anaesthesia (> 50 · 109/L), major surgery (> 50 · 109/L) or neurosurgery (> 100 · 109/L). However, these recommendations are based largely on clinical experience, and the evidence base is limited (25).
Other treatment modalities
Drugs can affect the production and destruction of platelets. In the critically ill, steroids may be administered if a low platelet count is suspected to have an immunological origin.
Conclusion
Thrombocytopaenia is common in critically ill patients. It often results from an interplay between several different pathophysiological mechanisms that together reduce the production of platelets and/or increase their consumption or destruction. The most significant cause of thrombocytopaenia is sepsis/infection. Thrombocytopaenia is a marker for poor prognosis and increased mortality risk in intensive care patients. A thorough consideration of the medical history and the clinical course, together with targeted laboratory diagnostics, are often sufficient to identify the underlying cause. This is necessary with a view to determining the likely prognosis and providing appropriate treatment. Treating the underlying cause should be the priority, with platelet transfusions being used mainly to avoid bleeding-related complications until the platelet count has been increased and haemostasis has been normalised.
