BCG instillation is a common procedure in Norwegian hospitals as part of the treatment of bladder cancer. We describe a patient who developed complications as a result of the procedure and a rare condition that proved difficult to diagnose.
A man in his seventies underwent intravesical instillation of bacillus Calmette-Guérin (BCG) as treatment for bladder cancer. The patient had a history of hypertension and had been a smoker for about 40 years. He had undergone treatment for oropharyngeal cancer five years ago, with no sign of recurrence. Three years ago, he underwent right upper lobectomy for non-small cell lung cancer; the following year small non-specific nodules were discovered in the right lower lobe, and these have been managed conservatively. His bladder cancer was diagnosed a year ago, and he has undergone two transurethral resections of the bladder (TUR-B) and nine BCG instillations.
During the outpatient BCG instillation in question (day 0), the catheterisation proved difficult and after a few hours the patient developed chills. He was therefore taken to the Emergency Department the same day. The patient had dysuria and pollakiuria, and had observed blood in his urine, but this was not unusual for him after instillations. In Accident and Emergency (A&E), his general condition was considered to be good; his chills had subsided, and his tympanic temperature fell from 38.1 °C to 37.5 °C without paracetamol. CRP was 6 mg/L (reference range <5 mg/L), leukocytes 5.3 · 109/L (3.5–10.0 · 109/L), and liver enzymes were normal. Urine dipsticks showed leukocytes 2+, nitrite negative, protein 3+, blood 3+ and ketones 1+. The results were considered to be as expected after BCG instillation, and the patient was sent home.
BCG consists of live attenuated Mycobacterium bovis (1), which is used both as a tuberculosis vaccine and as treatment for non-invasive urothelial bladder cancer. Intravesical instillation of approximately 5 · 108 bacteria in 50 mL of saline, retained in the bladder for two hours, stimulates the immune system to attack cancer cells in the bladder, and is highly effective with an 80 % cure rate (2). Non-serious complications, such as pollakiuria and dysuria up to four hours after an instillation, are common; haematuria somewhat less so. Low-grade fever and malaise lasting up to 48 hours after an instillation are also not uncommon, and can be considered a marker of a good anti-tumour response (1).
On day 2, the patient returned to A&E because of a gradual deterioration in his general condition. The dysuria and pollakiuria had persisted and he had developed a slight dry cough. He had also recorded a temperature of 38.6 °C. While in A&E, his blood pressure was 117/73 mm Hg, heart rate 97 beats/min, SpO2 97 % in room air, respiratory rate 16 breaths/min and temperature 37.4 °C. He was perceived to be weak, but not dyspnoeic. Auscultation revealed normal respiratory sounds, and his abdomen was soft but not tender. CRP was 111 mg/L, leukocytes 5.0 · 109/L and platelets 92 · 109/L (145–390 · 109/L). Liver enzymes were now abnormal, with AST 199 U/L (15–45 U/L) and ALP 124 U/L (35–105 U/L). Chest X-ray was normal. The patient was admitted to a urological ward and was given trimethoprim/sulfonamide (Bactrim 80 mg/400 mg, two tablets × 2) to cover both urinary tract and respiratory tract infections.
Over days 3–7, the patient’s general condition fluctuated with a fever of up to 39 °C. He was unsteady when walking, and felt that the unsteadiness had come on suddenly the evening of his most recent BCG instillation and had been unchanged ever since. PCR (amplification of DNA) of a nasopharyngeal specimen was negative for common respiratory pathogens. Bacterial cultures from the throat and nasopharynx showed normal flora, blood cultures were negative, and small amounts of white staphylococci were detected in the urine. CRP fell to 47 mg/L, but liver-bile enzymes increased, with AST 236 U/L, ALT 202 U/L (10–70 U/L), gamma-GT 527 U/L (15–115 U/L) and ALP 300 U/L. The patient denied abdominal pain. Abdominal ultrasound showed normal findings with no signs of cholestasis. Tests for autoimmune hepatitis and viral hepatitis were negative. The patient’s antibiotic was switched to amoxicillin 500 mg tablets × 3 to rule out an effect of trimethoprim/sulfonamide on transaminases, but with no improvement. BCG-induced hepatitis was now considered a possible explanation, and the patient was transferred to the medical department on day 6.
Serious complications of intravesical BCG immunotherapy are rare and are reported in fewer than 5 % of cases (3). Fever above 39.4 °C is the most common complication (2.9 %), followed by significant haematuria (1 %), granulomatous prostatitis (0.9 %), pneumonitis and/or hepatitis (0.7 %) and sepsis (0.4 %). In cases of hepatitis, jaundice is often present and granulomas may be found on liver biopsy (2). The complications are associated with bacteria gaining access to the blood and lymphatic system as a result of damage to the urothelium in the bladder wall (1). Risk factors are thought to include urinary tract infection, traumatic urinary catheterisation during instillation, early instillation after TUR-B, and underlying immunosuppression (1). There is disagreement as to whether serious complications are the result of a genuine invasive infection or whether they reflect a hypersensitivity reaction (1). A number of studies have been unable to detect the bacteria, even in cases where there is strong clinical suspicion of BCG infection. Combination therapy with anti-tuberculosis drugs and glucocorticoids is therefore common.
On day 7, we decided to try a course of prednisolone 60 mg in tablet form as treatment for a possible hypersensitivity reaction. A chest X-ray showed normal findings. As cystitis was a possible additional diagnosis, the patient’s antibiotic was changed back to trimethoprim-sulfonamide. By the next day his liver-bile enzymes were already improving, and upon discharge on day 11, AST and ALT were almost back to normal. We therefore decided against a liver biopsy. The patient still had a fluctuating temperature and slightly elevated CRP (16–38 mg/L), but overall was considered to be improving. His unsteadiness was attributed to orthostatism. The prednisolone dose was reduced to 40 mg and the patient was instructed to further reduce the dose by 10 mg every other day down to zero.
On day 18, the patient was re-admitted to the medical department. He had not recovered and felt so weak that he had difficulty walking. His appetite was significantly reduced and he had become short of breath. His blood pressure was 118/76 mm Hg, heart rate 100 beats/min, respiratory rate 18 breaths/min, SpO2 96 % in room air and tympanic temperature 38.1 °C. Abdominal examination was normal, apart from epigastric tenderness to palpation. Blood tests showed CRP 44 mg/L and sodium 130 mmol/L (137–145 mmol/L). Antibiotic treatment was not initiated on account of the modest increase in CRP, but correction of the hyponatraemia was started with 0.9 % NaCl infusion. It was speculated that the patient’s weakness might be the result of poor nutritional status the previous week.
Chest X-ray was normal, and was supplemented with CT thorax on day 19 to rule out an abscess as the cause of the fluctuating fever. CT thorax revealed innumerable micronodules in a bilateral miliary pattern (Figure 1). The pattern was most consistent with haematogenous spread, as seen in miliary tuberculosis and metastatic cancer. However, it was inconsistent with metastasis from adenocarcinoma of the lung. The possibility of systemic BCG infection therefore had to be considered. A urine sample taken on day 20 tested positive for mycobacteria in a PCR rapid test. Bronchoscopy on day 22 revealed no endobronchial pathology. Bronchoalveolar lavage (BAL) was used to obtain a fluid sample for mycobacterial and fungal diagnostic testing.
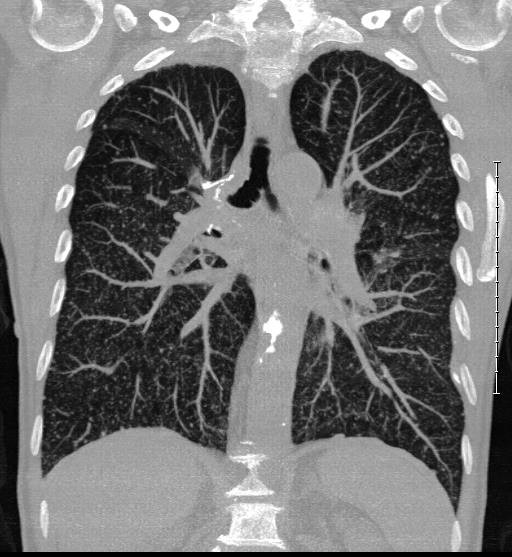
Figure 1 CT thorax on day 19 after BCG instillation shows a miliary pattern of innumerable micronodules in both lungs.
Diagnostic testing for tuberculosis entails the use of microscopy to detect acid-fast bacilli made visible by Ziehl-Neelsen staining, the use of PCR rapid testing with GeneXpert MTB/RIF Ultra to detect the Mycobacterium tuberculosis complex that includes BCG, and the use of the interferon-gamma release assay (IGRA), a blood-based test that measures the immune response to M. tuberculosis but not to BCG. Specimens are sent to Oslo University Hospital for culture. Low sensitivity has been reported for all microbiological diagnostic tests in cases of systemic BCG infection (2, 3). Whereas 42 % of tissue biopsies show bacterial growth, less than 5 % of patients are culture positive in blood, sputum, BAL fluid or cerebrospinal fluid. PCR tests are also rarely positive, with the highest frequency of positive results (10 %) seen with urine. However, detecting the bacterium in urine does not provide reliable support for BCG infection (local or systemic). This is because positive PCR results in urine have also been reported in the absence of complications at least seven days after instillation, while bacteria have been detected in bladder biopsies with PCR and Ziehl-Neelsen staining up to 24 and 16 months post-instillation, respectively (4, 5). Histological detection of acid-fast bacilli also has low sensitivity, of just under 70 % when a criterion of 104 bacteria per gram of tissue is used (2). As regards to diagnostic imaging, chest X-rays may be negative in up to a quarter of cases where a miliary pattern is seen on CT (5).
On day 23, the patient was evaluated by a neurologist on account of his unsteadiness, and brain MRI and lumbar puncture were also performed. Neurological examination revealed marked ataxia, both while sitting and standing, as well as pronounced nystagmus on lateral gaze in either direction. Tendon reflexes were normal for age. The patient had no neck stiffness or headache, and appeared alert and oriented to time, place and person. Brain MRI showed changes consistent with small, pre-existing lacunar infarcts in the basal ganglia, in addition to a possible slight enhancement of the dura mater. Lumbar puncture showed leukocytes <5 · 106/L (<5 · 106/L), total protein 0.22 g/L (0.15–0.50 g/L) and glucose 4.3 mmol/L (2/3 of serum glucose, normal). Because of the nystagmus and the fact that the ataxia was most pronounced in the lower extremities, as well as potentially poor nutritional status recently, we decided to administer thiamine infusions in the doses used for Wernicke’s encephalopathy, 500 mg × 1, and then 200 mg × 3 daily.
The patient had pronounced cerebellar deficits. However, neither areflexia nor ophthalmoplegia were present, which meant that Miller-Fisher syndrome (a variant of Guillain-Barré syndrome) was unlikely. There was a possible slight meningeal enhancement on MRI, which is not uncommon with infections. Such enhancement may also be seen after lumbar puncture, but in this case the MRI had been performed first. Normal cerebrospinal fluid may seem inconsistent with central nervous system inflammation, but there have been cases reported of miliary tuberculosis with central nervous system involvement with a clinical picture similar to that of our patient (6). Neuroantibodies were normal. None of the patient’s previous cancer types are known to give rise to paraneoplastic syndromes. Without a brain biopsy, it was not possible to rule out BCG infection as the cause of the ataxia, either via active infection with viable microbes or via a post-infectious immune response.
BAL fluid tested negative for DNA from the M. tuberculosis complex via PCR on day 23. The positive PCR from the urine sample was not considered sufficient evidence of systemic BCG infection, and following interdisciplinary discussions, it was decided not to begin tuberculosis treatment until a transbronchial biopsy had been performed. The patient’s ataxia appeared to improve as a result of the thiamine infusions, and eventually he was able to manage with only a walker for assistance, but it was somewhat unclear whether the improvement had in fact started before the infusions began. He reported few respiratory symptoms, but had an oxygen saturation of 93 % in room air. He was discharged home on day 35 with a transbronchial biopsy scheduled for two weeks’ time. He was advised to continue treatment with thiamine 100 mg tablets × 1 until readmission.
On day 44, another bronchoscopy was performed with bronchoalveolar lavage and transbronchial biopsy. The BAL sample tested negative for mycobacteria on PCR, and microscopy revealed no acid-fast bacilli. The patient still required a walker and now became short of breath upon exertion. His appetite remained poor. SpO2 was unchanged at 93 % in room air. Chest X-ray showed pronounced bilateral reticular pulmonary opacities. Because of the radiological and clinical deterioration, treatment for tuberculosis was started with rifampicin/isoniazid (Rifinah 300 mg/150 mg, two tablets) and ethambutol (Myambutol 400 mg, two tablets). On day 47, the biopsy results revealed necrotising granulomatous inflammation (Figure 2), but no acid-fast bacilli were seen upon Ziehl-Neelsen staining. The granulomatous inflammation observed in the biopsy reinforced the suspicion of BCG infection, however, and it was decided to continue treatment for six months. On day 54, mycobacterial growth was reported in the urine sample taken on day 20. The final results of cultures from the lung biopsy, BAL fluid, blood and cerebrospinal fluid were negative.
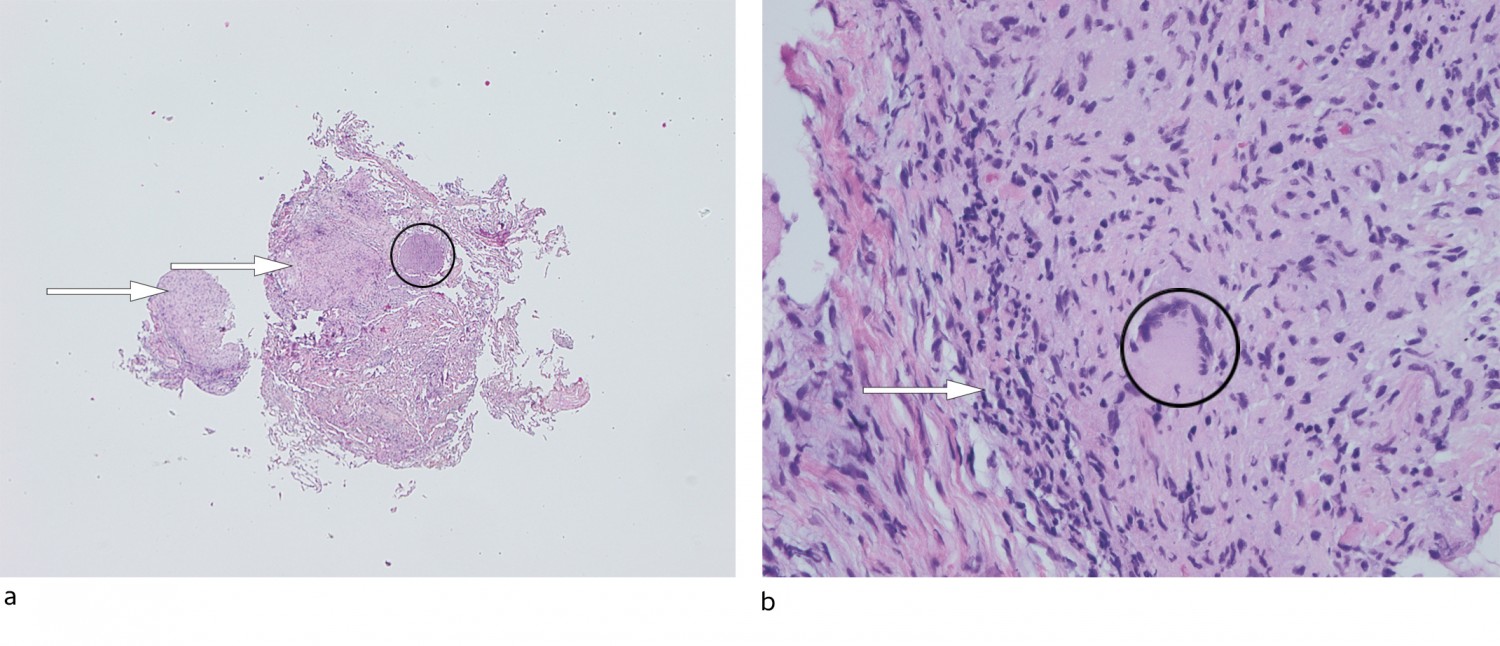
Figure 2 Lung biopsy, routine staining with haematoxylin and eosin. a) 40x magnification: lung tissue with granulomatous inflammation (arrows) can be seen with a few giant cells and focal necrosis (circle). b) 400x magnification: granulomatous inflammation (arrow) with a Langhans giant cell (circle).
Four months after completing treatment, the patient is in good general condition. He moves freely without a walker, but becomes short of breath easily. The pronounced infiltrates on the chest X-ray have cleared up, but small nodules remain in both lungs. CRP is normal, and the patient continues to be followed up.
Discussion
This case report illustrates the challenges associated with managing rare conditions in which the absence of a clear clinical picture or conclusive laboratory findings makes it impossible to make a firm diagnosis that can be used to guide treatment. In retrospect, antibiotic therapy could have been initiated earlier. This would be in line with the Norwegian Directorate of Health’s action plan for bladder and urothelial cancer, which states that treatment for tuberculosis should already be considered in the event of fever above 38.5 °C for more than 24 hours and concurrent general symptoms (7). Nevertheless, we found that there was little experience of treatment being started on the basis of this indication. The treatment is long-lasting and not without side effects, especially in the elderly, and as a general rule, treatment for a disease caused by M. tuberculosis should not be started before the bacterium has been detected.
We believe that the combination of a strong immune response plus active disseminated infection with M. bovis probably led to all of the organ manifestations seen in our patient. Most symptoms of active tuberculosis and leprosy (infection with Mycobacterium leprae) are known to result from a prolonged immune response dominated by cytokine production in antigen-presenting cells (2). One can speculate that in cases of systemic BCG infection following bladder instillation, there may be a brief period of haematogenous spread of viable M. bovis to multiple organs. However, the bacteria – which have relatively low pathogenicity – will be unable to survive outside the bladder for more than a few hours or days in the attenuated BCG form in an immunocompetent patient. Nevertheless, this may be sufficient to initiate a prolonged aseptic systemic inflammatory response syndrome (SIRS). This may explain why the bacteria cannot be detected in affected organs.
SIRS used to be seen frequently in the treatment of malignant melanoma, when intravenous or intratumoral BCG administration was used as immunotherapy (2). The treatment usually gave rise to intense SIRS, similar to that in our patient, but this was not considered to reflect a systemic BCG infection. However, there are reports in the literature of viable mycobacteria being detected in biopsies from affected organs, not just the bladder, several years after the last intravesical instillation. The longest reported interval between a final instillation and the emergence of complications is 17 years (epididymo-orchitis) followed by 11 years (spondylodiscitis) (1). Anti-tuberculosis drugs therefore continue to play a key role in the management of BCG infections and may themselves dampen the body’s strong immune response (8).
We chose to present this case to raise awareness among colleagues of an important complication of a relatively common procedure in Norway, which to the best of our knowledge has not previously been described in the Norwegian literature. Clearer guidelines for the management of this condition are required.
