Two young, healthy doctors were diagnosed with deep vein thrombosis 27 and 29 days, respectively, after receiving the AstraZeneca ChAdOx1 nCoV-19 vaccine. Both had a negative D-dimer test and a low Wells score.
A woman in her thirties and a man in his forties were vaccinated against COVID-19 with the AstraZeneca vaccine (ChAdOx1 nCoV-19). They were diagnosed with deep vein thrombosis 27 and 29 days after vaccination, respectively. Both were of normal weight and non-smokers with normal levels of physical activity. They had no underlying illnesses and had not been subject to trauma, surgery, infection, immobilisation or long-haul air travel in the preceding three months, or a COVID-19 infection. There was no known thrombophilia.
Patient 1
The patient was a female doctor in her thirties. Aside from hypothyreosis following Hashimoto’s thyroiditis in childhood and some lumbago issues, she had been otherwise healthy. She had no history of blood clots, including in connection with pregnancy and childbirth. She was treated with 150 µg of levothyroxine (Levaxin) daily.
The patient was given the ChAdOx1 nCoV-19 vaccine from AstraZeneca. The next night she woke up with intense back pain, and developed a fever and chills. These symptoms resolved within 36 hours.
Sixteen days after vaccination, she developed petechiae in two fingers. The Norwegian Medicines Agency had advised the public to seek urgent medical assistance if they developed symptoms within 14 days of vaccination (1), but as 16 days had now passed and the woman’s general health was good, she did not see a doctor or have any tests.
After a few skiing trips, however, three of her toenails turned blue and bruises appeared elsewhere on her body despite not experiencing any impact blows.
Twenty-seven days after vaccination, she awoke with pain in her right popliteal fossa and upper leg without prior trauma. She attributed the symptoms to a busy weekend shift at the hospital. The next day, the pain worsened. A routine hypothyreosis check-up to measure her thyroid hormones was supplemented with further analyses. Blood tests showed haemoglobin 12.7 g/dL (reference range 11.7–15.3), leukocytes 5.5 × 109/L (3.5–10.0), platelets 334 × 109/L (145–390) and D-dimer <0.4 mg/L (< 0.5). She used a simplified Wells score, which showed 0, and concluded that deep vein thrombosis was unlikely.
The next day, she still had severe pain in her popliteal fossa and upper leg, and asked a colleague in the Department of Radiology to carry out an ultrasound examination to rule out deep vein thrombosis. Thrombus was then revealed in the distal popliteal vein (Figure 1).
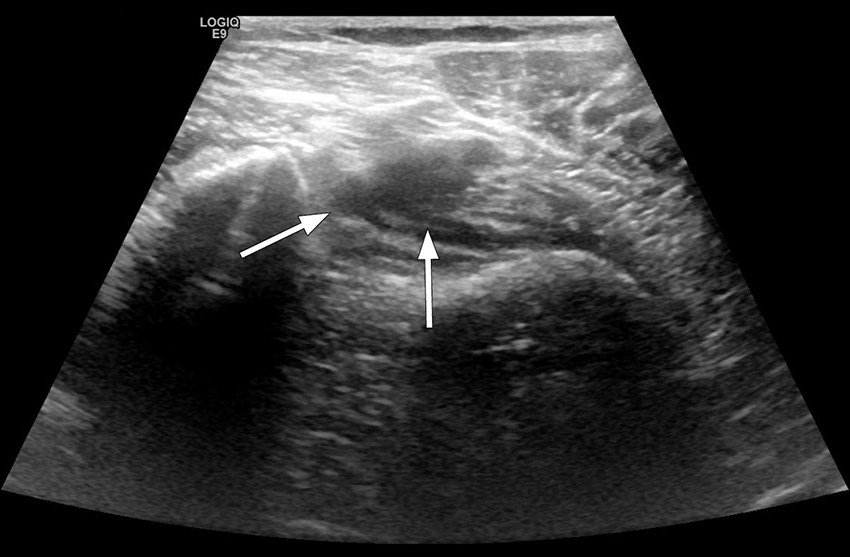
Figure 1 Ultrasound image of right lower extremity, patient examined with legs hanging over the edge of the bed. Distal popliteal vein shown upon compression. A sickle-shaped shadow is seen in the centre of the image, and the vein cannot be compressed, as is the case for intraluminal content.
She was treated with rivaroxaban for three months, 15 mg × 2 for three weeks, followed by 20 mg × 1. One week after confirmation of deep vein thrombosis and initiation of anticoagulant therapy, a head MRI was taken and platelet antibodies were measured in connection with a survey of adverse effects of vaccines. Both examinations revealed no pathological findings. The case was reported as an adverse effect in the registration system for healthcare workers, Melde.no.
Patient 2
The patient was a previously healthy male doctor in his forties who did not use regular medication. Ten hours after vaccination with ChAdOx1 nCoV-19, he developed a brief fever and chills, but continued to feel unwell the next day. Over the next 3–4 days, he experienced joint pain and exhaustion. Light exertion also triggered dyspnoea, which lasted approximately one week.
Ten days after vaccination, he felt slight proximal discomfort in his left lower leg. He interpreted this as a muscular issue, alternatively a Baker’s cyst. The symptoms gradually intensified, and after 16 days he observed a slight swelling. However, this receded after a few days. Twenty-four days after vaccination, he took blood tests because he was still experiencing discomfort and because of the media reports about thrombi/bleeding as a possible adverse effect of the vaccine he received.
He did not have a headache. Blood tests showed haemoglobin 15.9 g/dL (13.4–17.0), leukocytes 5.5 × 109/L (3.5–10.0), platelets 303 × 109/L (145–390) and D -dimer <0.4 mg/L (<0.5). The Wells score was 0. On the same day, he contacted a radiologist, who performed an ultrasound examination but could not confirm the doctor’s suspicion of deep vein thrombosis. Six days later, 29 days after vaccination, he experienced pain in his left lower leg. A new ultrasound examination revealed deep vein thrombosis. He was treated with Apixaban 10 mg × 2 for the first seven days, followed by 5 mg × 2 for three months. The case was reported as an adverse effect on Melde.no.
Discussion
Two patients without underlying risk factors developed deep vein thrombosis, 27 and 29 days, respectively, after receiving the AstraZeneca ChAdOx1 nCoV-19 vaccine. A clear association has now been established between the vaccine and what is referred to as vaccine-induced immune thrombotic thrombocytopenia (VITT), a condition with thrombosis in unusual sites, thrombocytopenia and bleeding (2–4). However, cases reported had occurred within 14 days of vaccination.
The clinical picture with VITT is unique and dramatic, and the two patients discussed here did not have the same clinical picture. The level of platelet antibodies was not elevated in the patient who was tested for this. Neither of the patients had headaches, one had petechiae in two fingers, bruising and three blue toenails, but otherwise no symptoms of coagulation disorders. Both had normal platelet counts. As the cause of VITT is unknown, other thromboembolic conditions or bleeding cannot be ruled out as being part of the overall clinical picture, although this may be less likely.
D-dimer is formed by the degradation of cross-linked fibrin. Elevated D-dimer levels are a non-specific finding that can be seen in conditions such as infection, trauma, pregnancy, cancer, disseminated intravascular coagulation and after surgery. D-dimer, on the other hand, has a high negative predictive value, i.e. normal concentrations rule out deep vein thrombosis with more than 90 % certainty in ambulant patients with clinical symptoms of thrombosis (5). In the cases in question, the D-dimer level was not elevated, and the diagnosis of deep vein thrombosis was made by ultrasound examination.
Both patients had low Wells scores, which means that, according to guidelines (6), they should not have been referred for an ultrasound examination. Most flow charts for patients with deep vein thrombosis would recommend discharge without treatment. The two patients would probably have gone undiagnosed had they not been doctors themselves and in a position to ask a colleague to perform an ultrasound examination. Very thought provoking.
COVID-19 infection is known to increase the risk of thrombosis (7). One study showed that Wells scores were unreliable for predicting pulmonary embolism in COVID-19 patients: 4 out of 12 patients with pulmonary embolism had a Wells score of 0 (8). Another study showed that high Wells scores were as common in COVID-19 patients without pulmonary embolism as in COVID-19 patients with pulmonary embolism (9). It is conceivable that the situation is also similar post-vaccination as for COVID-19 infection. Perhaps Wells scores do not have the same negative predictive value after vaccination? Symptoms of thrombosis after vaccination should probably be followed up somewhat more closely than indicated by the standard procedure.
In a scenario of extensive vaccination with new vaccines, it is vital that both patients and doctors are vigilant. Suspected new, unexpected or serious adverse effects must be reported.