Abdominal pain is common in pregnancy and can be caused by anything from physiological changes to life-threatening illness. Non-obstetric conditions can be challenging to diagnose due to atypical symptoms as well as the tendency to attribute any changes to the pregnancy. There must also be a strong clinical indication for diagnostic imaging that could prove harmful to the fetus.
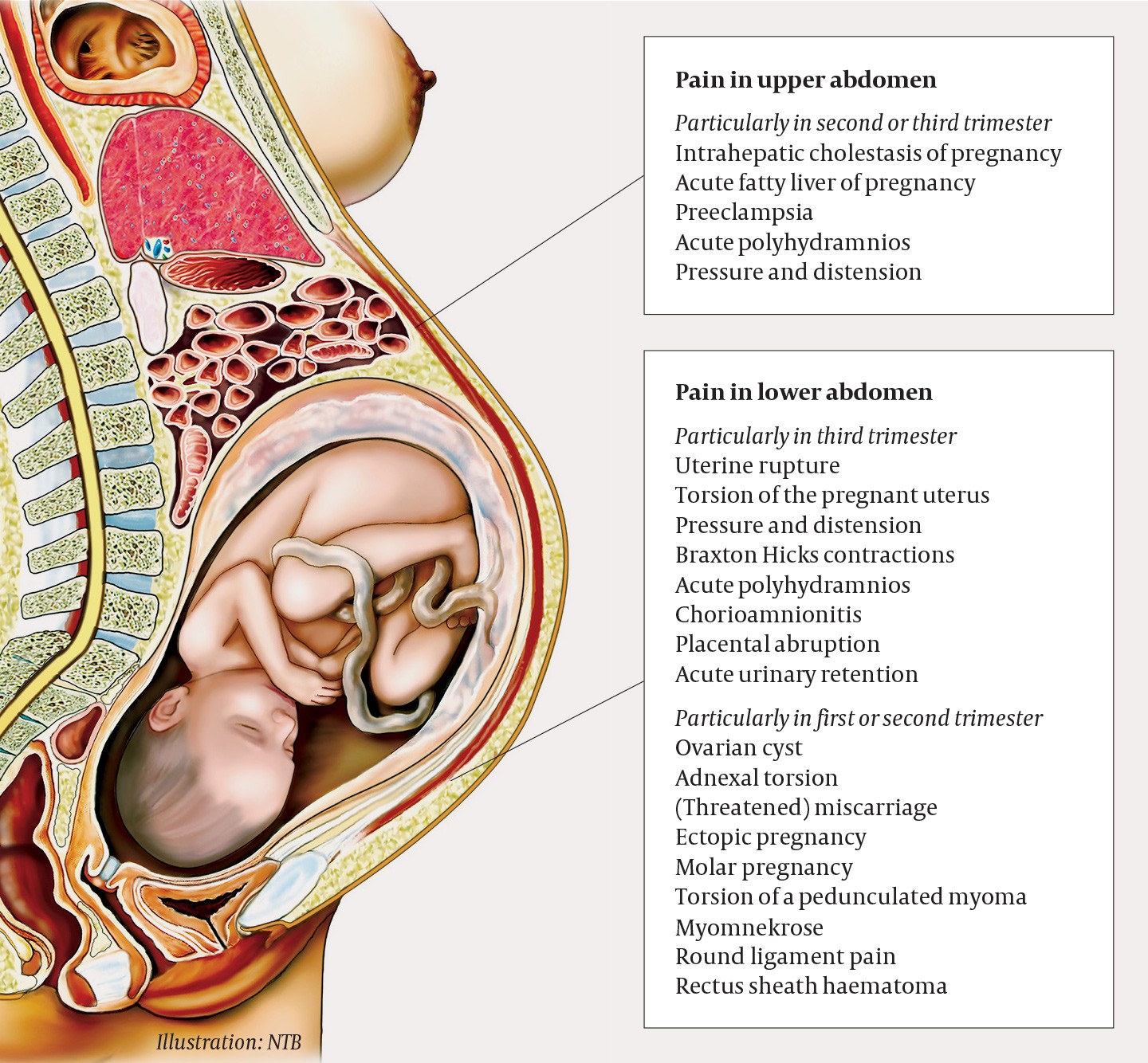
Figure 1 Gynaecological diagnoses to consider in cases of acute abdomen in pregnancy. The risk assessment will depend on gestational age, clinical findings and biochemical results.
A primigravida was admitted to the maternity ward in week 34 of pregnancy with non-traumatic acute abdomen. She was previously healthy and on no regular medications. The pregnancy had been largely uncomplicated, but the woman had been examined four weeks earlier because of abdominal pain, and had been diagnosed with mild hydronephrosis of the right kidney. Paramedics notified the maternity ward that the woman would be arriving with an immediately life-threatening condition; this call was made on the basis of her intense pain. The woman’s extremities were cold and clammy, she had mild tachycardia (105 beats/min) and a high respiratory rate (40/min), but otherwise normal vital signs. The ambulance arrived at the hospital 45 minutes after the pain had started. The midwife moved the patient to a delivery room, summoned the doctor on duty and examined the woman. There were no signs of contractions, latent labour or vaginal bleeding. Measurement of vital signs on arrival showed that she was afebrile with blood pressure of 127/100 mm Hg and a heart rate of 90 beats/min. Her respiratory rate was not measured on arrival and no urine sample was obtained, but her antenatal health card stated that a urine sample a few days earlier had been normal. Cardiotocography (CTG) showed normal fetal heart rate, and preliminary abdominal ultrasound showed a living fetus with normal, spontaneous movements. The placenta also appeared normal on ultrasound, with no evidence of retroplacental haematoma.
Contractions and latent labour, which should always be considered as potential causes of abdominal pain in pregnant women, were first ruled out. In pregnant women who have severe abdominal pain without being in labour, potentially serious pregnancy-related complications must then be considered, such as preeclampsia, placental abruption and uterine rupture. Preeclampsia (blood pressure ≥ 140/90 mm Hg with at least one other new sign of maternal organ/placental damage after gestational week 20) occurs in 2–3 % of pregnancies in Norway (1). Risk factors for preeclampsia include pre-existing kidney disease or a high body mass index, diabetes, hypertension, maternal age over 40 years and primiparity (2). Abruptio placentae – complete or partial separation of the placenta before the baby is born – has an incidence of 0.3 % (1, 2). Risk factors for placental abruption include essential hypertension, low body mass index, previous intrauterine fetal death, smoking, use of cannabis or cocaine, and maternal age < 20 years or > 40 years (3, 4). Pain caused by placental abruption is often accompanied by heavy vaginal bleeding. Uterine rupture is a rare diagnosis in women with no prior history of uterine surgery, and in such women the incidence is 0.005–0.02 % (2, 5). However, the risk increases after surgery on the uterine wall: after one previous caesarean section, the risk of rupture is 0.2–1 %, and after two or more caesarean sections, the risk increases to 2 %. This is why caesarean section is recommended for women with two previous caesarean sections.
Our patient had no history of abdominal surgery and no other risk factors for placental abruption. The fetus remained unaffected, unusual in both placental abruption and uterine rupture. The possibility of a somewhat atypical onset of severe preeclampsia should always be considered, and the woman did have mild hypertension on arrival. Further observation, with repeated measures and blood tests, is necessary to rule out HELLP (preeclampsia-related organ failure which manifests as haemolysis, elevated liver enzymes and a low platelet count). Ovarian torsion is another serious and acute gynaecological condition that must be considered in cases of acute abdomen, but the growth of the uterus further along in the pregnancy helps protect against torsion by giving the ovaries less space to rotate around their own axes. Our patient had no back pain or macroscopic haematuria, which made urinary tract pathology less likely. She was afebrile, and the pain had hyperacute onset and was not accompanied by nausea or vomiting. This argues against acute surgical abdomen, although ileus, appendicitis and pneumoperitoneum could not be ruled out.
Transabdominal ultrasound revealed abundant extrauterine free fluid. In the lower right quadrant, a fluid pocket measuring 7 cm in diameter was observed. The patient appeared in great pain and the abdomen was peritonitic with tenderness and rebound tenderness in all quadrants. Both placental abruption and uterine rupture are clinical diagnoses that cannot be ruled out by ultrasound. The patient was still haemodynamically stable with blood pressure 111/54 mm Hg, SpO2 100 % and heart rate 105 beats/min, and the fetus remained unaffected. Test results and clinical features were consistent with intra-abdominal bleeding, and the gynaecologist on duty quickly decided to deliver the baby by caesarean section combined with a simultaneous exploratory laparotomy. Surgery was scheduled to begin within 20 minutes. As the woman had not arrived via Accident and Emergency, blood samples were requested some 20 minutes after her arrival, and the results were not available prior to the start of surgery.
It is important to note that pregnancy can mask a severe haemorrhage of up to 1200–1500 mL, because pregnant women are often young with no comorbidities and therefore compensate well for blood loss, while the pregnancy itself gives rise to a physiological hypervolemia. Blood pressure and heart rate can thus be within normal ranges until the woman has lost more than 1.5 litres of blood, and her uterus and physiologically distended abdomen can hide signs of peritonitis and distension caused by intraabdominal fluid. It was decided to perform the caesarean section under general rather than spinal anaesthesia, owing to the rapid onset of general anaesthesia, with the possibility of a longer operating time than with spinal anaesthesia, and because it would allow the patient to be monitored and treated more easily in the event of major blood loss.
The woman and her spouse were continually updated on the situation. Upon arrival in the operating theatre, she was still haemodynamically stable, and the caesarean section itself began six minutes later. When the peritoneum was opened, a large volume of blood and numerous blood clots were seen. The uterus was opened according to standard procedure with a transverse incision in the lower uterine segment, and clear amniotic fluid spilled out. The baby was delivered two minutes after the start of the procedure, with Apgar scores of 1–5–7 and weighing 2970 g. Only the results of venous blood from the umbilical cord were recorded, which showed pH 7.02 and base deficit of 11.58 mmol/L. An acute hypoxic event is defined as pH < 7.0 with base deficit > 12 and Apgar score < 5 after 5 minutes with signs of moderate/severe encephalopathy. The infant did not fulfil these criteria. The child was ventilated for 4–5 minutes and was breathing spontaneously after 2–3 minutes. A normal heart rate > 100 was observed after 1 minute. Chest X-ray showed dense white lungs and neonatal respiratory distress syndrome. Additional blood samples were drawn and showed pH 7.21, pCO2 6.7, base excess −7, lactate 6.4. There was no sign of placental abruption. Inspection of the front and back of the uterus revealed no signs of rupture.
A large volume of blood was observed in the abdomen; it was subsequently estimated that about 2.5 litres of blood and clots were present prior to delivery. Immediately after opening the peritoneum, and while the baby was being retrieved from the uterus and the umbilical cord cut, the surgeons began manual compression of the aorta and summoned reinforcements in the form of the on-call specialist in obstetrics and gynaecology, as well as a gastrointestinal surgeon and a vascular surgeon. A longitudinal incision of the abdominal wall had to be made in addition to the initial transverse incision, to provide an adequate view of the surgical field. The vascular surgeon quickly arrived and clamped the left common iliac artery. The pelvis was packed and the upper part of the abdomen was inspected without any source of bleeding being identified. The vascular clamp was loosened and an injury was detected in the left uterine vein at the level of the cervix. Satisfactory haemostasis was achieved by vessel ligation. The patient experienced a brief and minor fall in blood pressure to 90/60 mm Hg in the period between opening the peritoneum and clamping of the aorta, but was otherwise haemodynamically stable with a consistent blood pressure of 100/60 mm Hg. Due to heavy blood loss, she received 1500 mL of red cell concentrate (SAG), 800 mL Octaplas and 375 mL of platelet concentrate. The estimated total blood loss was approximately 4700 mL.
Forty-eight hours after surgery, the patient was transferred from the intensive care unit to a standard ward, but was readmitted to intensive care the same day with abdominal pain and paralytic ileus. She returned to the ward 24 hours later, after a new clinical assessment found no evidence of new-onset intra-abdominal bleeding, and epidural pain relief proved effective. After 13 days, she was able to return home in good health without the need for any further surgical intervention. The infant initially received neonatal respiratory support with continuous positive airway pressure, but was discharged from the neonatal intensive care unit after ten days with no follow-up required.
Discussion
It was decided to deliver the baby immediately because uterine rupture and placental abruption could not be ruled out. These are clinical diagnoses of high severity that require emergency intervention. The fetus had a gestational age of over 34 weeks, which made the decision to deliver more straightforward in terms of fetal viability and maturity. In addition, the size of the uterus at week 34 means that delivery would be essential prior to any other abdominal surgery. If the event had occurred earlier in the pregnancy, and it had been possible to obtain a sufficient intra-abdominal status without delivery, then continuing the pregnancy might have been an option. This scenario was described by Ginsburg et al. in a woman in week 26 of pregnancy (6). Their patient underwent a laparotomy on vital indication with ligation of the uterine vein, and later gave birth by vaginal delivery at term to an infant of normal birthweight.
If abdominal pain occurs in connection with a major trauma or accident, pregnant women should be evaluated in Accident and Emergency in the same way as other patient populations, but healthcare professionals must additionally consider the possibility of serious complications such as fetal injury, fetomaternal transfusion, premature birth, premature rupture of membranes, uterine rupture and placental abruption. In such cases, any essential diagnostic imaging should be performed on the same indication as for non-pregnant women. Peritoneal lavage can also be performed, but it is recommended to use open technique and to limit the procedure to the upper part of the abdomen. It is important to be aware that pregnant women have a physiological respiratory alkalosis at the end of pregnancy, and that D-dimer levels in blood may be slightly elevated (0.13–1.7 µg/mL in the third trimester) and the platelet count slightly reduced (146–429 × 109/L), relative to non-pregnant women (7).
Our patient received a primary transverse abdominal incision. If the pathogenesis had been known in advance, the patient could instead have benefited from a primary longitudinal incision, thereby avoiding a T-incision associated with poorer wound healing and larger scars. We felt that uterine rupture was the most likely diagnosis, and in such cases a transverse incision will generally provide the abdominal access required for effective surgical intervention. However, a longitudinal incision is much more practical for inspecting the upper abdomen, and also makes it easier to perform effective aortic compression.
The child had a low Apgar score and reduced blood flow in the umbilical vein, but did not meet the criteria for an acute hypoxic event. The low Apgar score was probably the result of several factors, but it is likely the mother’s hypovolemia contributed to short-term placental insufficiency. The fetal CTG was normal prior to the mother being transferred to the operating theatre, although the signal quality was somewhat poor.
Acute spontaneous haemoperitoneum in pregnancy
Acute spontaneous haemoperitoneum in pregnancy is a rare condition that was first described in 1778. Since then, around 100 case reports have been published. The condition has often proved difficult to diagnose, and it can be challenging to locate the exact bleeding focus. Prior to 1950, the diagnosis was usually made on the basis of autopsy, and in 1950 the mortality rate was around 49 % (8). The condition typically begins with acute pain and hypovolemic shock in the absence of an identified bleeding focus (9). The diagnosis is rarely made prior to laparotomy, with the condition usually misinterpreted preoperatively as placental abruption. A review of case reports has shown that 50 % of diagnoses are in primipara (10). In 75 % of cases, the rupture occurs in the broad ligament of the uterus. The haematoma can also dissect into the retroperitoneal space and thereby give rise to a slightly different clinical picture with more diffuse symptoms that often include back pain. Rupture of the ovarian vein has also been described. Ginsburg et al. found that 61 % of cases of spontaneous bleeding occur antenatally, 19 % during labour, and 21 % postpartum.
The pathogenesis is unclear. Hodgkinson and Christensen’s hypothesis from the 1950s is that the physiological increase in blood flow to the uterus and ovaries leads to dilation of the venous plexus, predisposing veins to spontaneous rupture. Circulatory studies have shown that venous pressure in the femoral vein increases by a factor of 2–3 during pregnancy, and animal experiments have revealed that this change is only partly attributable to the pressure exerted on the veins by the growing uterus. The ovarian and uterine veins also lack venous valves, rendering them more vulnerable to pressure changes. A sudden change in intravenous pressure has been described as a possible causal mechanism. Bleeding could then be triggered by mechanisms that increase intra-abdominal pressure, such as the increased physiological demands of pregnancy, as well as coughing, defecation, coitus, and the second stage of labour (9).
All healthy pregnant women will develop an abundant venous plexus around the uterus, but nevertheless acute bleeding is very rare, which suggests there may be other causal mechanisms. Gravity and the size of the uterus contribute to increasing venous stasis over the course of the pregnancy. Hormonal changes in pregnancy also affect the venous system independent of uterine size (11), as does the increase in plasma volume (12). Increasing progesterone levels lead to relaxation of smooth muscle cells surrounding veins, while increasing oestrogen levels promote relaxation of collagen fibres. This reduction in vascular tone leads to vasodilation. Combined with the increase in venous capacity, this results in insufficient valves that do not close completely,-contributing to venous stasis and an increased risk of varicose veins and thrombosis. The increase in oestrogen affects the arterial wall as well as the synthesis of prostaglandins and nitric oxide; the latter is also a potent vasodilator (13).
Several studies have suggested an association between acute spontaneous haemoperitoneum in pregnancy and endometriosis (10). Endometriosis is the growth of endometrial tissue outside the uterus, usually on the peritoneum of the abdomen and pelvis, and on the ovaries and fallopian tubes. The condition is associated with chronic pain and infertility. Patients with endometriosis often find that their condition improves during pregnancy; however, the condition also gives rise to a chronic inflammatory state within the abdomen, which makes blood vessels more fragile and therefore more prone to rupture. The lesions also make the tissue less elastic and the vessels more vulnerable to stretching as the uterus grows. In addition, existing endometrial implants will be stimulated by endogenous progesterone during pregnancy, resulting in the formation of decidual tissue that is richly vascularised and carries an independent risk of spontaneous bleeding (14–16). Decidua is the term used to describe the tissue that is formed during pregnancy by the mucosal lining of the uterus and the maternal placental tissue. Acute spontaneous bleeding from the uterine vessels may occur throughout pregnancy and postpartum, but is most common in the third trimester (16).
Bleeding from decidual tissues was first described as a cause of death in a pregnant woman in 1957 (17). A review of autopsy reports from 1929 to 2006 found two maternal deaths, three fetal deaths and two neonatal deaths among the ten cases identified (15). Maternal mortality in this rare condition has fallen significantly since the 1950s, but fetal mortality remains at 31 % (6).
In our patient, there was no anamnestic or clinical evidence to suggest endometriosis. We therefore conclude that the vein ruptured spontaneously in the absence of a clearly identified cause.
The patient has subsequently had another pregnancy, with delivery via an uncomplicated elective caesarean section at week 39. The infant was born healthy with a normal birthweight for gestational age.
