Tularaemia can be challenging to diagnose. Here we present a case illustrating how pulmonary tularaemia can be an important radiological differential diagnosis to lung cancer.
A man in his fifties contacted his general practitioner on account of fever, reduced general condition and a dry cough. An initial PCR test on a pharyngeal swab was negative for SARS-CoV-2. He was given an appointment three weeks after symptom onset; upon presentation at the medical centre, he still had profuse night sweats and a dry cough, but was now afebrile. He was a former smoker with a history of about 20 pack-years. He also had epilepsy, which was well-controlled with anti-epileptic drugs, but was otherwise in good health. Clinical examination was normal. Laboratory tests showed CRP of 48 mg/L (reference range <5) and leukocytes within the reference range. Chest X-ray showed patchy opacities in the right superior and middle lobes.
At a follow-up appointment four weeks after symptom onset, he was still having night sweats but his dry cough had resolved. His CRP level was 22 mg/L.
Previous pneumonia was considered a possible diagnosis. However, on account of the persistent night sweats, the general practitioner referred the patient for a CT scan of the thorax and abdomen. The images showed significant mediastinal lymphadenopathy and two consolidations in the right lung. The lung masses and lymph nodes both showed signs of central necrosis (Figure 1). The findings were described as suspicious of malignancy, with enlarged adrenal glands suggesting possible adrenal metastases. The patient was therefore referred to the local hospital on the lung cancer pathway.
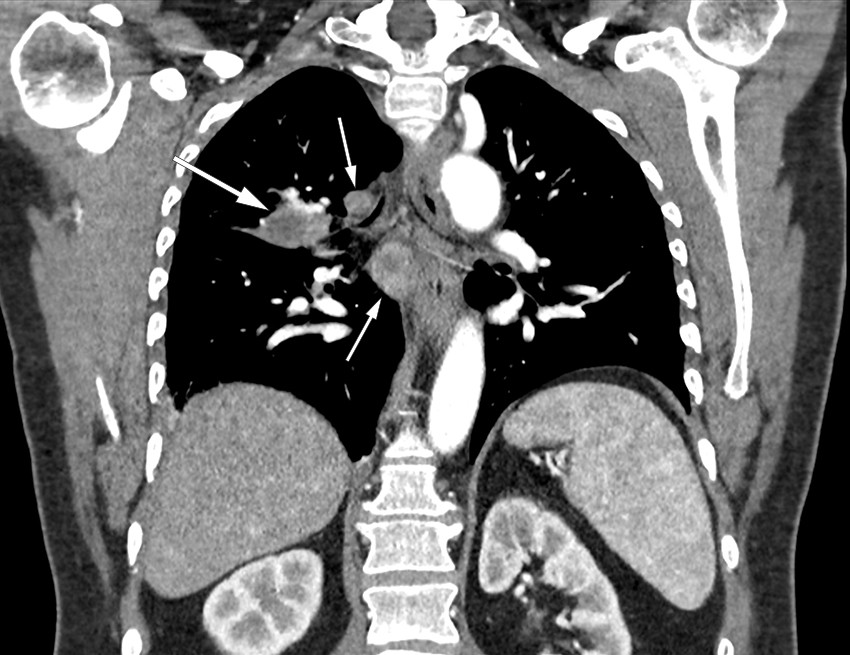
Figure 1 Coronal CT thorax with contrast in a patient with pulmonary tularaemia showing extensive lymphadenopathy (small arrows) and a consolidation near the right hilum (large arrow), both with central necrosis.
Upon assessment at the local hospital six weeks after disease onset, the patient was anxious owing to the radiological findings, but otherwise felt well. He had no cough or night sweats. He now reported that his weight had normalised after having decreased by 10 kg during his illness. Clinical examination was normal, and laboratory tests showed CRP levels of 11 mg/L. The significant clinical improvement could indicate an infectious aetiology. In recent years we have seen similar imaging findings in patients with pulmonary tularaemia. It turned out that the day before the patient became ill, he had tidied up an old woodpile in which mouse droppings had been present. Serological tests were positive for Francisella tularensis IgM and IgG, confirming a diagnosis of pulmonary tularaemia. He was treated with ciprofloxacin 500 mg × 2 orally for ten days. A CT scan of the adrenal glands showed findings consistent with adrenal adenomas. At an appointment four months after symptom onset, he was fully recovered and the chest X-ray was normal.
Discussion
Tularaemia is a zoonosis caused by the bacterium Francisella tularensis. There are three different biotypes of Francisella tularensis, but only F. tularensis subsp. holarctica is found in Norway. Tularaemia is usually fatal in hares, while rodents can survive for an extended period and become reservoirs of infection. Tularaemia is divided into six types on the basis of clinical manifestation and site of inoculation: ulceroglandular, glandular, oculoglandular, oropharyngeal, pulmonary and typhoid. Pulmonary tularaemia is usually caused by inhalation of F. tularensis. The infectious dose is very low: with aerosol transmission, 10–25 bacteria are sufficient to cause disease. The incubation period is usually 3–5 days, but can vary from 1–21 days (1–3). As we were unable to identify any other potential sources of exposure besides the woodpile, we assume that our patient had an unusually short incubation period and had become infected the day before symptom onset. Aerosols containing F. tularensis had probably been generated from rodent excreta within the woodpile. Activities such as hunting, outdoor pursuits, farming, logging and the restoration of old houses are known routes of transmission. It is therefore important to obtain a detailed history of activities and leisure travel (1, 4, 5). It is also important to educate the population about disease prevention. It is recommended to wear gloves when handling rodents or dead hares. Mouse droppings should be removed using a damp cloth rather than a broom. Respiratory protection should also be considered when handling materials in which rodent excreta may be present (3, 5).
In Norway, tularaemia is notifiable and cases must be reported to the Institute of Public Health’s Surveillance System for Communicable Diseases. Norway is among the countries with the highest incidence of tularaemia, and reported cases are showing an upward trend. Cases are seen throughout the whole of Norway, but most frequently in Eastern Norway and in Trøndelag county. There are no official statistics on the prevalence of the various clinical manifestations, but oropharyngeal and ulceroglandular tularaemia are said to be the most common types (3). However, pulmonary tularaemia accounted for almost half of all cases of tularaemia in a recent observational study from Innlandet county, published by our group (4). Pulmonary tularaemia is probably underdiagnosed and should be considered more frequently as a differential diagnosis (3). The disease is most common in the autumn, when extra vigilance is therefore required (4, 6).
Pulmonary tularaemia typically gives rise to fever and general malaise, and sometimes also to respiratory symptoms (1, 2, 4, 6). In our experience from around 40 cases in recent years, patients have often had reduced general condition for several weeks. This has also been described in several case reports (5, 7). Tularaemia can be detected by means of serology, PCR or bacterial culture. Serological testing is the cornerstone of diagnosis owing to its widespread availability and high sensitivity. However, it is important to be aware that the antibody response – both IgM and IgG – is late, often occurring two to four weeks after symptom onset (1, 2, 8). We therefore recommend that testing is repeated in cases with negative serology, but where there remains clinical suspicion of the disease.
On chest X-ray, pulmonary tularaemia typically manifests as opacities and hilar lymphadenopathy. CT thorax generally reveals enlarged lymph nodes and multiple consolidated, nodular opacities, often in the periphery. A characteristic sign of the disease is the presence of central necrosis in lymph nodes and opacities, as was seen in our patient (4, 6, 9). The difficulty of distinguishing pulmonary tularaemia from malignancy, particularly lung cancer, is recognised in the literature (4, 5, 7). It is therefore important that radiologists are informed of any clinical signs of infection.
It is stressful for patients to be referred on to a lung cancer pathway. Unfortunately, these patients often undergo unnecessary, risky and costly procedures such as PET-CT, CT-guided biopsy, bronchoscopy and endobronchial ultrasound (4). We believe the key to rapid and effective diagnosis is to consider pulmonary tularaemia as a differential diagnosis at an early stage, especially if the patient lives in or has visited a rural area. Serological testing can be ordered in both the primary and specialist healthcare services.
In cases where diagnostic imaging and medical history suggest pulmonary tularaemia, serology is a straightforward way of confirming the diagnosis.
